MRD:vg3217 2ndyearcomp
1. Mathematical definition of transition state
This part is good.Sw2711 (talk) 13:21, 16 May 2019 (BST) A transition state can be defined as a saddle point on the potential energy surface, i.e. a point having coordinates where:
- The gradient of the potential energy is 0 with respect to each coordinate. Thus, is a stationary point of V:
- The potential surface is concave with respect to one coordinate and convex with respect to the other (the transition state is a local maximum on the minimum energy path between reactants and products). Thus, the second derivative test discriminant of V at is less than 0:
Saddle points can therefore be mathematically distinguished from local extrema, in which case the above discriminant is greater than 0 and:
for minima
for maxima
2. Estimate of transition state coordinate
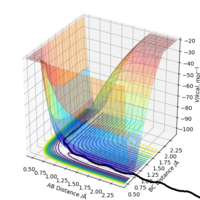
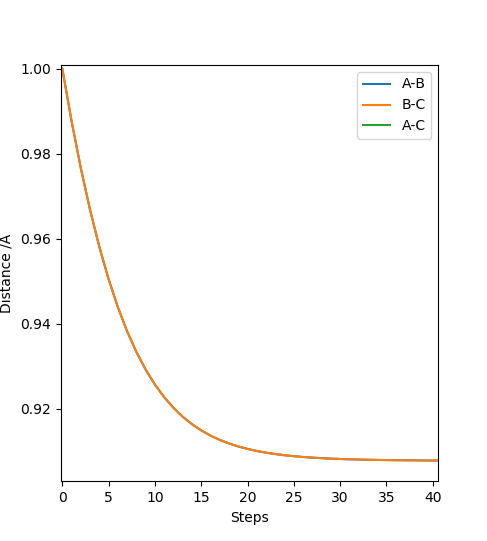
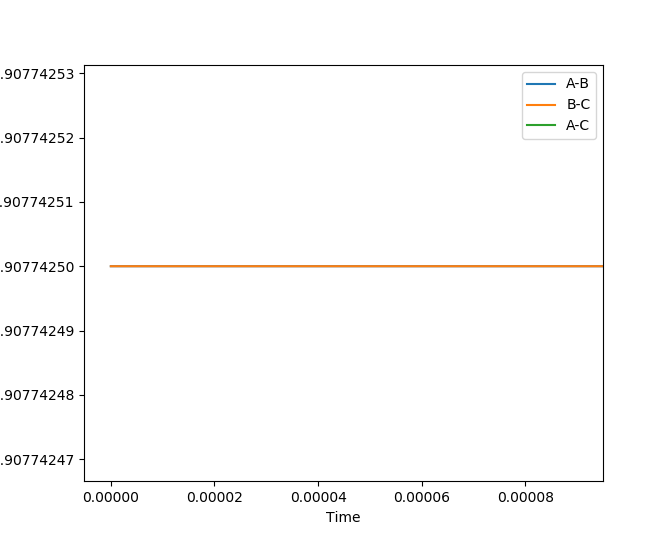
Very good way of identifying the TS.Sw2711 (talk) 13:22, 16 May 2019 (BST) The H + H-H reaction should have a symmetrical potential energy surface, since the reactants and products are identical. Therefore, the transition state should be a point having coordinates , where . The point can therefore be found using an MEP simulation choosing any equal initial coordinates, such that the system will come to rest at the TS. Thus, has been estimated as 0.9077425 A. This was confirmed using a dynamic simulation of internuclear distance versus time, where no oscillation can be observed.
 |
 |
3. MEP vs dynamics simulations
Good.Sw2711 (talk) 13:23, 16 May 2019 (BST) When applying a minimum energy path simulation, the momenta of all particles are reset to 0 at each time step. As a result, the particles will always follow the gradient of the potential energy surface until they reach a stationary point.
Placing the particles in the transition state with no initial momentum, as above, will result in no trajectory, as this is already a stationary point (with no gradient). Offsetting the particles from the transition state will result in them moving to the starting materials or products side of the surface (depending on the direction of the offset), where they will remain stationary, as these are local minima.
Additionally, MEP simulations also result in no bond vibration, since particles will come to rest at the equilibrium bond distance.
 |
 |
4. Reactive and unreactive trajectories
It follows that kinetic energy alone does not predict whether a reaction will take place. Rather, the distribution of energy between translation and vibration should be taken into consideration.
Transition state theory makes two central assumptions:
- The energy levels of the reactants follow the Bolzmann distribution.
- Once the reactant has reached the transition state, on a trajectory towards the product(s), it will not revert to the reactant region.
This part is concise, good in general good. You are sort of getting there. But it is better to state whether TS theory supports or against your observation. Also, do we have a Boltzmann distribution in our system? .Sw2711 (talk) 13:26, 16 May 2019 (BST)
5. The F - H - H system
5.1. Potential energy surfaces
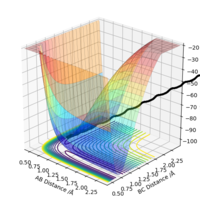
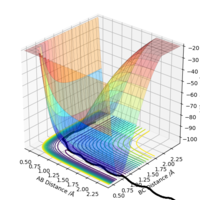
By inspection of the potential energy surfaces of the F-H-H system, it can be determined that F + H-H is an exothermic reaction (products have lower potential energy), while F-H + H is endothermic (reactants have lower potential energy). It should follow that the F-H bond is stronger than the H-H bond.
| F + H-H | F-H + H |
|---|---|
 |
 |
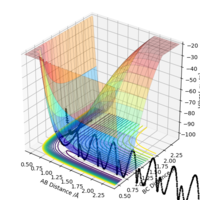
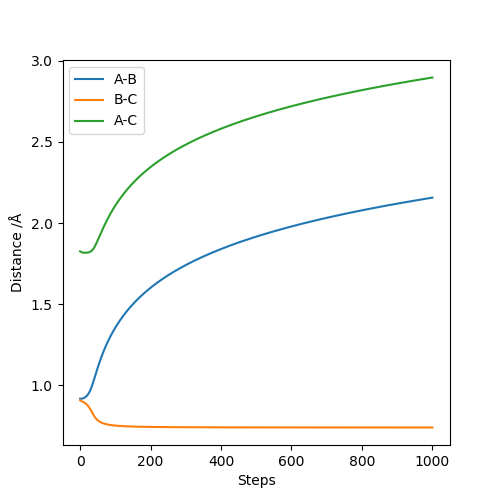
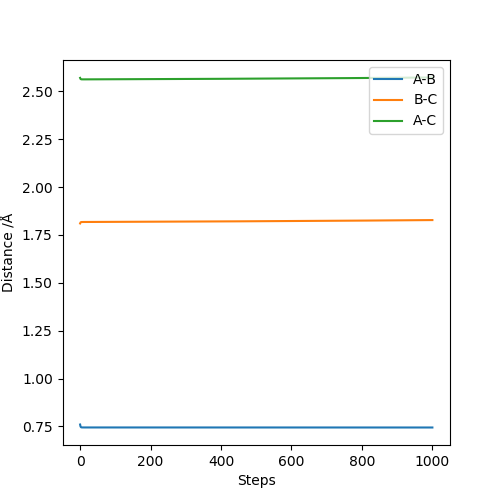
Applying Hammond's postulate, since F + H-H is an exothermic reaction, the transition state is closer in energy and, therefore, in structure to the products. Are you sure? I think in Hammond's postulate, an exothermic reaction has a TS closer to reactants..Sw2711 (talk) 13:30, 16 May 2019 (BST)Approximate transition state coordinates have been empirically determined using an MEP simulation rHH=0.76 A and rHCl=1.81 A and confirmed using a dynamic simulation presenting low, constant oscillation.
 |
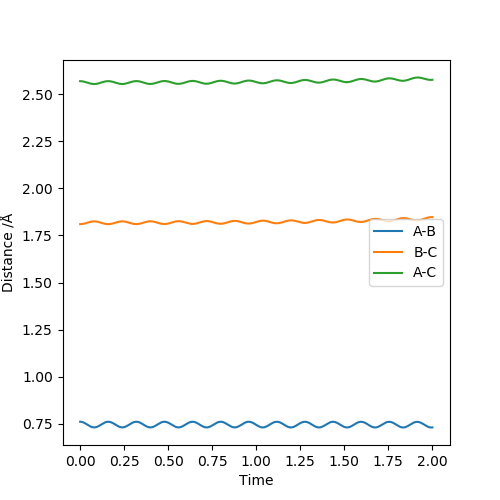 |
The activation energy for each reaction was determined as the difference between the transition state energy and the energy of the system in each initial state:
ETS= -103.664
EA exothermic= 0.176
EA endothermic= 30.196
I think you can do slightly better in finding the TS. But it's okay. What's more important, you need to tell me how do you find the energies of TS, reactants/products. Also don't forget to include the units. Should be KCal/Mol Sw2711 (talk) 13:33, 16 May 2019 (BST)
5.2. Reaction dynamics
Potential energy surfaces may be classified as attractive or repulsive, depending on the degree of bond breaking and bond formation in the transition state, compared to the products and reactants. Considering how the H-H bond in the F-H-H activated complex is relatively close to the starting material, this potential energy surface is attractive and presents an early transition state. Conversely, the potential energy surface of the reverse reaction is repulsive and presents a late transition state.

The influence of the attractive or repulsive nature of the potential energy surface and the required type of vibrational and translational energy can be visualised: if the trajectory oscillates with the required amplitude and frequency to pass through the transition state and "reflect" off the potential energy surface, the products will be formed. Otherwise, it will either not overcome the activation barrier or revert to the starting material.
From the simulated reaction path, most of the reaction energy seems to be transferred to vibration in the product; this could be observed by infrared chemiluminescence, as the product relaxes to the vibrational ground state.
The reverse reaction could then follow the same trajectory, but in the opposite direction: an excited vibrational state would be more effective to overcome the higher activation barrier, while most of the energy of the product would be translational.
I think this part is about the conservation of energy? You are sort of describing it. But are there any figures which demonstrate the idea in a more straightforward way? Are you describing the trade-off between translational and vibrational energy or potential energy and kinetic energySw2711 (talk) 13:43, 16 May 2019 (BST)
For the Polanyi's rule, I think you also sort of mentioned it. But I would expect you to do a bit more to investigate the statement. And it's better to clearly label where your answers are for which question.Sw2711 (talk) 13:43, 16 May 2019 (BST)