MRD:ti1918
Thomas Imperato MRD Report
This report has some strong areas, but there are sections which could have been improved with more information or even the support of figures. Mys18 (talk) 14:39, 1 June 2020 (BST)
Exercise 1:
Question 1:
The transition state on a potential energy surface diagram is mathematically defined by the derivative ∂V(ri)/∂ri equalling zero. It can be identified by starting trajectories near its approximate location and seeing whether they form products or revert back to reactants; if you change the geometry one way and it forms products and then change it the opposite way and it forms reactants then that is the transition state. It can be distinguished from other local minima as on the surface plot it is the point where all gradients vanish.
First sentence is right, what is this point known as and what actually is it, non mathematically. There is a better mathematical method to differentiate between a local minimum and the TS, it involves the second derivative. Check out what you would expect the answer for the second derivative to be for a local minimum and the TS. Mys18 (talk) 14:11, 1 June 2020 (BST)
Question 2:
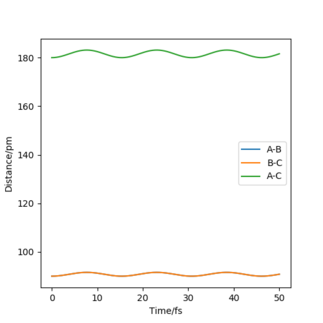
The best estimate for the transition state position was 90 pm as the oscillations in Figure 1 were the smallest at this distance. Also, as I change the trajectory so that one atom has a tiny momentum of 0.1, a hydrogen molecule is formed and the other atom leaves the molecule shown in Figure 2.

That's a good estimate, how did you calculate this, was it using Hammond's Postulate? Mys18 (talk) 14:14, 1 June 2020 (BST)
Question 3:
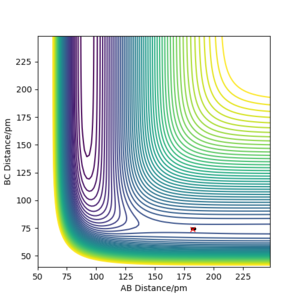
The MEP and dynamic plots differ as the MEP plot does not show vibrational energy of the oscillating hydrogen molecule. Furthermore, the MEP plot internuclear distance begins to plateau as the number of steps increases whereas the dynamic plot follows a relatively linear path.
Good, it does not show it because it does not take the vibrational motion into account. You could support your finding with a contour plot which neatly illustrates the differences in the trajectories. What about the velocities... this will affect the path in both trajectories.Mys18 (talk) 14:18, 1 June 2020 (BST)
Question 4:
We can conclude from Figure 3 that even if the total energy is greater than the activation energy a reaction won't always occur as the translational and rotational also needs to be in the correct composition.
Question 5:
From the results in Figure 3 we can see that the total energy does not always predict the outcome of the interaction. So therefore experimental data will differ from the theoretical as the theoretical assumes that whenever the energy is greater than the activation energy a reaction will occur. But the experimental data won't always agree with this as in reality (experimental) the distribution of energy to vibrational etc components is more important.
Figure 3 plots are slightly too small. You reactivity answers are correct. Good, so even though the activation energy barrier can be overcome, why exactly does it not go from Reactants to products only. Consider looking up transition state theory assumptions and the key words may be useful in forming your answer eg, barrier recrossing, tunneling. With the reaction rate you can hopefully answer that with a greater insight to the assumptions in mind. Mys18 (talk) 14:24, 1 June 2020 (BST)
Exercise 2:


Question 1:
From Figure 4 we can see that the reaction between HF and H is endothermic as the potential energy of the system becomes more positive due to the large energy input required to break the H-F bond overpowers the release of energy when the smaller H-H bond is formed. In Figure 5 we see that the HH and F reaction is exothermic as the potential energy of the system becomes more negative due to the release of energy when the strong H-F bond is formed. It all revolves around the fact that the H-F bond has greater bond energy than the H-H bond.
Excellent answer! You can even go one step further and support your answer, literature search bond energies for H-F and H-H to show what you found is exactly true (we would expect that right, with our knowledge on bonding). Mys18 (talk) 14:28, 1 June 2020 (BST)
Question 2:
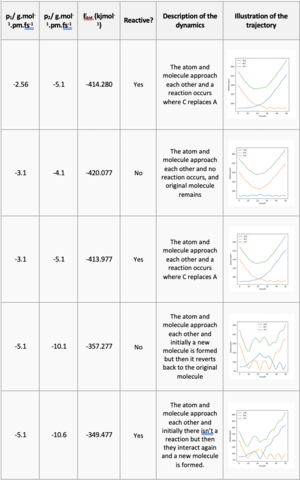
The approximate transition state for the reactions was 74 pm distance between the hydrogens and 182 pm between the hydrogen and fluorine. In Figure 6 we see that there is only a single dot on the plot using a MEP calculation, a classic property of a transition state.
those values look good. Did you expect these values from what you know of Hammond's postulate? Mys18 (talk) 14:30, 1 June 2020 (BST)
Question 3:
In order to find the activation energy you must find the difference between the potential energy of the transition state and the potential energy of the reactants. The potential energy of the transition state was found to be -433.945 Kj/mol by using a MEP calculation at the TS point. The potential energy of the reactants that form a hydrogen molecule is -558 Kj/mol so the activation energy is 124.055 Kj/mol. For the reverse reaction that forms a HF molecule has reactants with a potential energy of -434.245 Kj/mol so the activation energy is 0.300 Kj/mol.
They look good again, nicely remembered units. Mys18 (talk) 14:31, 1 June 2020 (BST)
Question 4:
The release of the reactant energy is carried out by the mechanism known as chemiluminescence, where light energy is emitted in response to the exothermic chemical reaction. After the reaction the product is in a quantum excited state and returns to the ground state by emitting a photon. This can be analysed by a technique called IRCL which provides data so that we can tell if the energy released is more vibrational or rotational depending on how intense the IRCL feedback is. To calculate the actual value of the energy released calorimetry should be carried out which is a straight forward experimental procedure.
Question 5:
The distribution of vibrational and rotational is very important in affecting the efficiency of the reaction as even with a large enough potential energy, if the vibrational and rotational aren't correct then a reaction can't occur. According to Polanyi's rules, if the reactant is similar to the transition state then the reaction is exothermic and vibrational energy is very important in allowing the reaction to occur. If the product is similar to the transition state then the reaction is endothermic and the vibrational energy is not that vital for the reaction to take place.
May be best described in terms of a 'early/late transition state'. So it is based on how the barrier position will determine the different energy demand. Endothermic rxn with early TS mainly determined by translational energy (even with low vibrational energy). Those with late TS vibrational energy is more effective in converting reactants to the products. Check out what an exothermic reaction and the late/early TS differs. Mys18 (talk) 14:38, 1 June 2020 (BST)
