MRD:sq111714052019
Molecular Reaction Dynamic Wiki Sicong Qiu
Exercise 1 : the H-H-H system.
On a potential energy surface diagram, how is the transition state mathematically defined? How can the transition state be identified, and how can it be distinguished from a local minimum of the potential energy surface?
It is the point where the second derivatives of both V(r1) and V(r2) are 0. At this point r1 is equal to r2. A local minimum will only have the first derivative of either V(r1) or V(r2) equal to 0, and their second derivative will not be 0.
Okay, however it does not clearly answer the question. In words think about what a TS state is and so it would make your mathematical explanation of a minimum more relevant. If the second derivative is not zero, then what will it be (positive or negative)? Mys18 (talk) 16:53, 20 May 2019 (BST)
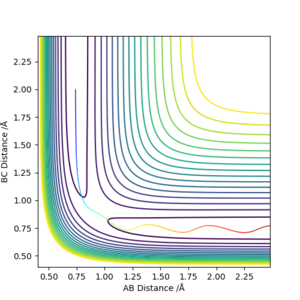
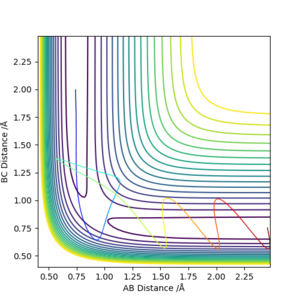
Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” plot for a relevant trajectory.
The transition state position is approximately 0.908 angstrom. The internuclear distances look to be stabilised, as it is sitting right on the saddle point. The distances of A-B and B-C in the initial simulation also intercept at ~0.91 angstrom.
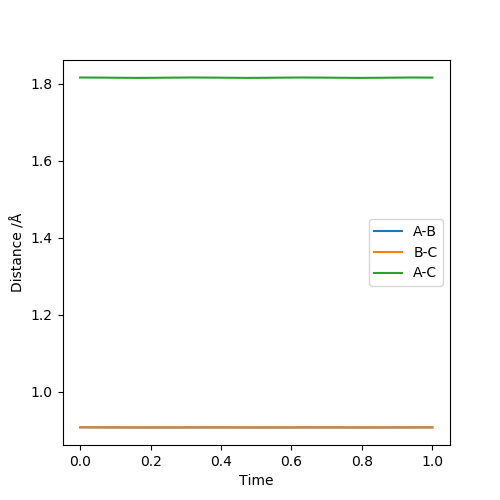
Good inclusion of units! Indeed, the internuclear distances are constant. Mys18 (talk) 17:57, 20 May 2019 (BST)
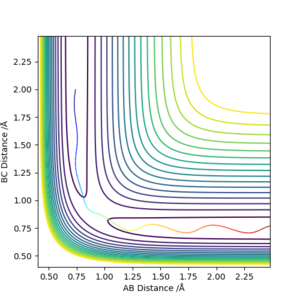
Comment on how the mep and the dynamic trajectory differ.
Dynamic graph shows the inertial motion and oscillation of the molecule, which is shown as the internuclear distance is oscillating.
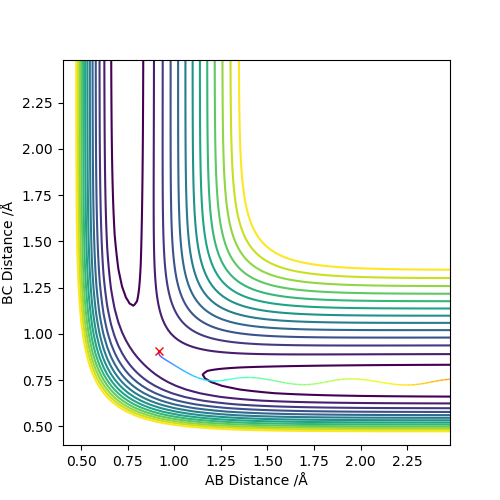
MEP graph shows infinitely slow motion, so the inertial motion and oscillation is ignored. The internuclear distance is a lot more smooth.

Because momenta is reset to 0 after each step, kinetic energy is lost and the total energy is not conserved. The total energy using dynamic method is -99.119 kcal/mol, and the total energy using MEP method is - 103.869 kcal/mol. The difference is the kinetic energy.
Very good! What could you also say about vibrational motion wrt. the MEP/Dynamic method? Mys18 (talk) 17:08, 20 May 2019 (BST)
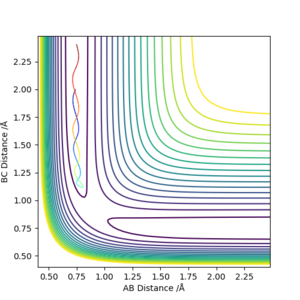
Reactive and unreactive trajectory
Conclusion
To achieve reaction, the particles must have kinetic energy above a threshold. However, even with enough energy, there's a chance that energy transfer will happen instead of reaction.
Good observations. For the first row perhaps we ought to consider atom C approaching molecule AB and so, we are looking at whether or not we form BC. REACTION 4 you are correct, but lets dwell on the "somehow" part; we see reactants crossing the TS to form products, following this we see reactants forming again...what do you think has happened? Mys18 (talk) 17:14, 20 May 2019 (BST)
Main assumptions in the transition state theory and how they affect the predicted rate in comparison the experimental value.
There're a few assumptions being made in the transition state theory1:
1. Nuclear motion and electron motion are separate, similar to the Born-Oppenheimer approximation.
2. Maxwell-Boltzmann distribution is applied to the reactant molecules.
3. Molecules that have crossed the transition state cannot reform reactants.
4. In the transition state, motion along the reaction coordinate may be separated from other motion and treated classically as a translation.
5. Even in the absence of equilibrium between the reactant and the product, the transition states that are becoming the product follows the Maxwell-Boltzmann laws.
However:
1. In our simulations, there're examples that molecules with sufficient energy that have crossed the barrier reform the reactant. With this in mind, the realistic reaction rate is likely to be lower than predicted.
2. For a very short lived transition state, the activated complexes may not have enough time to reach the equilibrium according to Maxwell-Boltzmann laws before turning into products.
3. Particles may behave according to quantum mechanics instead. For a reaction with low energy barrier, particles without sufficient energy may tunnel through the barrier and react.
Perfect! Consider the third assumption for the question above. Do you know what the proper term is when reactants --> products then go back over the TS --> reactants? Mys18 (talk) 17:16, 20 May 2019 (BST)
Exercise 2: the F-H-H system.
Energetics and the bond strength of the chemical species.
Bond strength in kJ/mol
H-H: 436 H-F: 567
In the F + H2 reaction, a H-H bond is broken and a H-F bond is formed. The reaction is exothermic with -131 kJ/mol.
In the H + HF reaction, a H-F bond is broken and a H-H bond is formed. the reaction is endothermic with 131 kJ/mol.
Very good, nice to see you have found literature values for bond strengths. Using your chemistry knowledge of bonding, why is the H-F bond stronger? Mys18 (talk) 17:18, 20 May 2019 (BST)
Locating the transition state
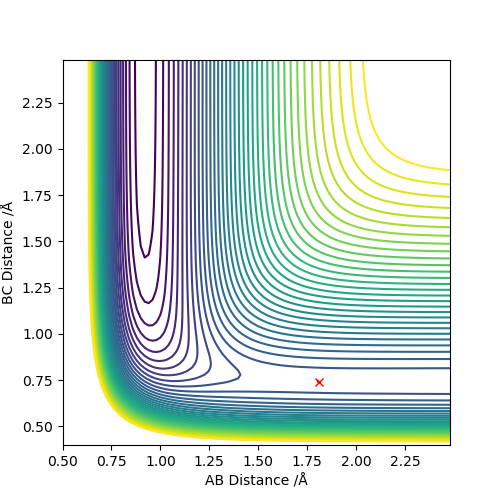
F + H2 system
rHF = r1 = 1.810, rHH = r2 = 0.745. At these distances, the internuclear distances can stabilise.
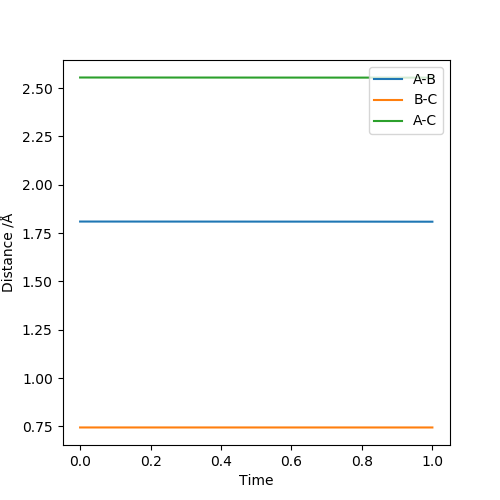
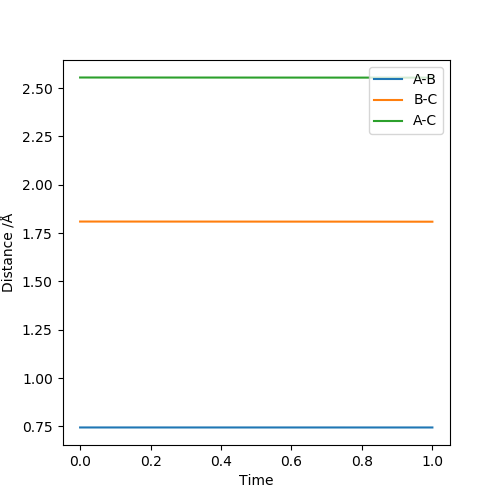
H + H-F system
rHH = r1 = 0.745, rHF = r2 = 1.810. At these distances, the internuclear distances can stabilise.
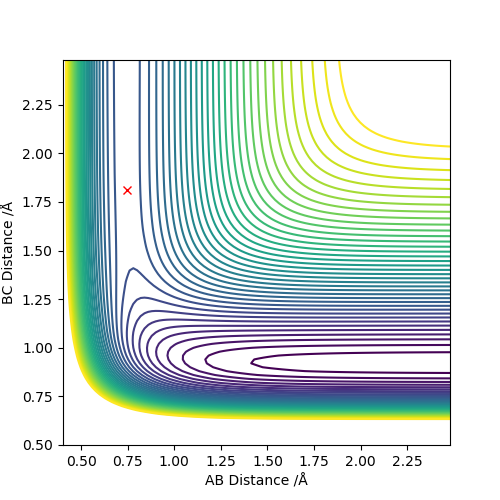
The activation energies
A = F, B = H, C = H
By increasing rAB by 0.01 from rts, the reaction is pushed toward the F + H-H side. The activation energy is -103.75-(-103.99) = 0.24 kcal/mol
By decreasing rAB by 0.01 from rts, the reaction is pushed toward the H + H-F side. The activation energy is -103.75-(-133.91) = 30.16 kcal/mol
Well done in showing how you arrived at the activation energies with the correct units. Mys18 (talk) 17:20, 20 May 2019 (BST)
Mechanism of the release of energy in the F + H-H reaction and experimental approach
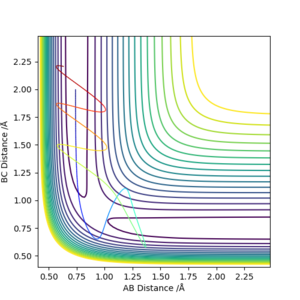
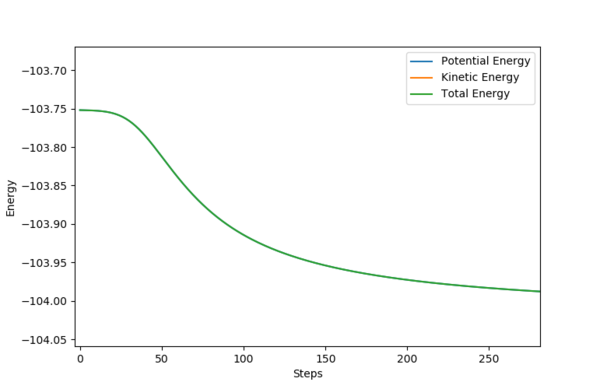
The following setup is proved to be a reactive trajectory.
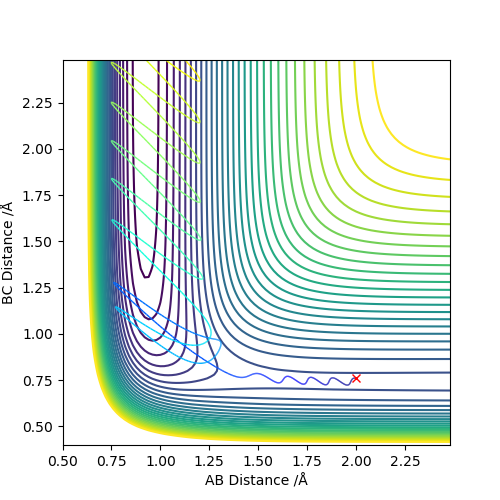
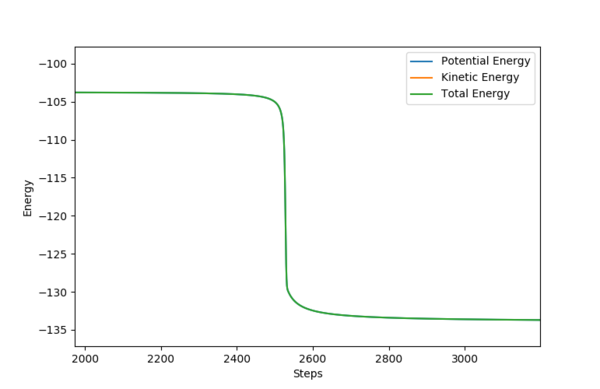
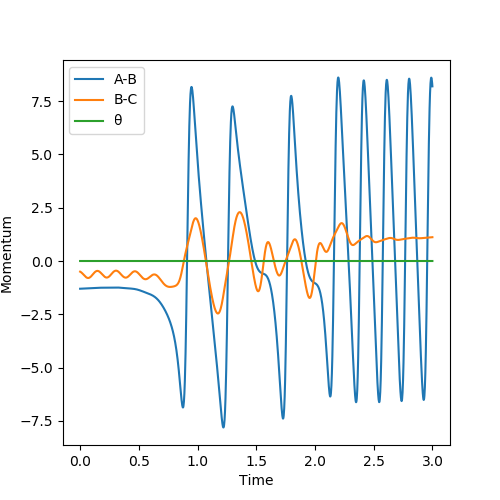
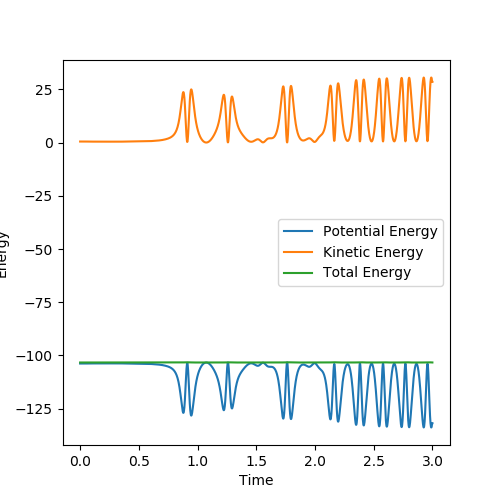
It is clear that the initial momentum leaves the product in a hot vibration state, with an increase in momenta and kinetic energy. The momenta and energy vs time graph confirm this. A subsequent drop in potential energy is observed, which makes sure that the total energy is conserved.
Vibration hot state can be detected using UV-Vis spectroscopy. In an adiabetic environment, the temperature of the system will also increase, which can be measured by a thermometer.
Interesting ideas, would have been nice see you expand on your suggestions. How else are vibration frequencies detected? Mys18 (talk) 17:22, 20 May 2019 (BST)
The position of the transition state and its relationship with vibration and translation energy.
According to Hammond's postulate, because the F + H-H reaction is exothermic, it should have an early transition state that mostly resembles the reactant. On contrary, the H + H-F reaction is endothermic, and its transition state should occur late in terms of reaction coordinates and mostly resemble the product.
The following setup produces a reactive trajectory for the H + H-F reaction.
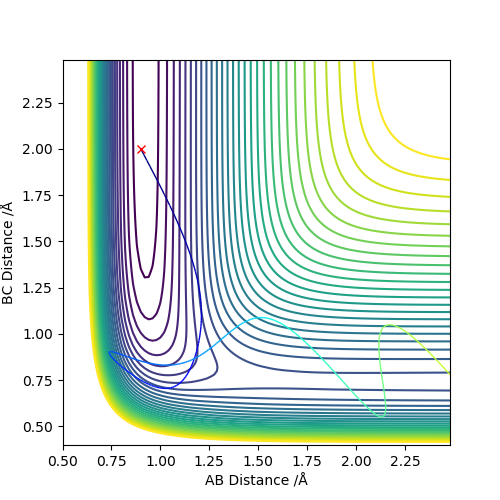
In these systems, vibration energy is represented by the intra-molecular momentum of the diatomic, and translation energy is represented by the momentum between the approaching atom and the diatomic.
In the exothermic F + H-H system, converting translation energy into vibration energy results in a failed trajectory, while having more translation energy and less vibration energy do not hinder the reaction.
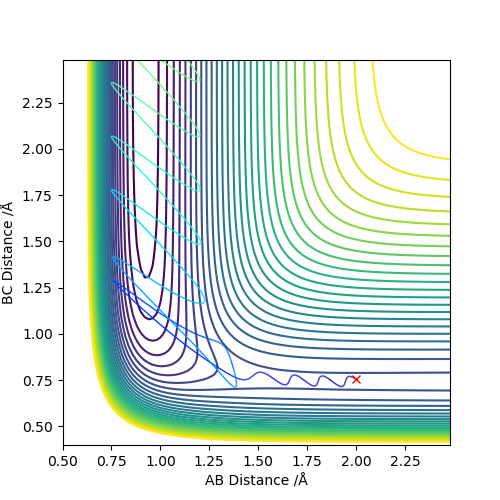
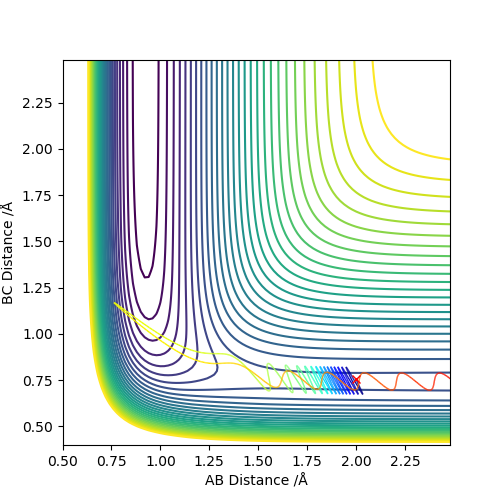
In the endothermic H + H-F system, it is the opposite. Vibration energy is preferred over translation energy.
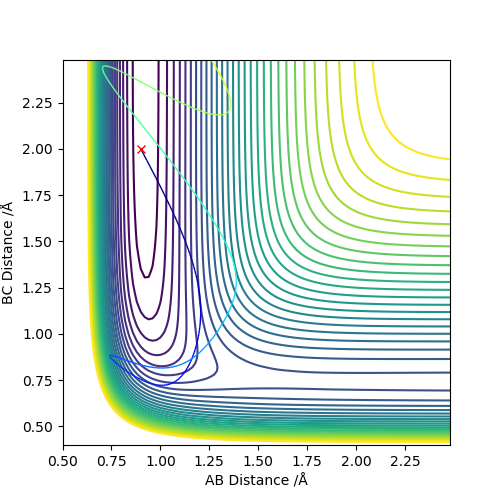
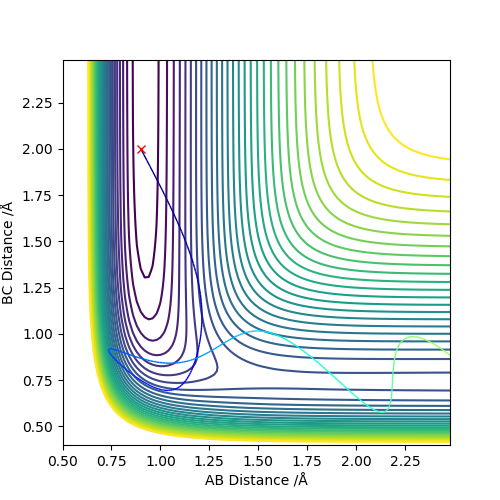
As a result, it seems that an early transition state/exothermic reaction favours translation energy to provide the relatively small 'push' over the kinetic barrier, while a late transition state/endothermic reaction focuses more on having more vibration energy on the reactants themselves to get over the thermodynamic barrier.
Nice work linking Polanyi's rule to your computational findings. Overall, good work and nicely laid out report. You answered all questions with understanding, with a few areas that could have had more detail to make things clearer. Good job :) Mys18 (talk) 17:28, 20 May 2019 (BST)
Refenrence
[1] J. I. Steinfeld, J. S. Francisco, W. L. Hase, Chemical Kinetic and Dynamics, Prentice-Hall, New Jersey, 1998