MRD:sk716-2
Exercise 1: H + H2

Potential energy vs. trajectory surface
A plot of potential energy vs. trajectory can be used to investigate the reaction with the above reactants. The gradients along both axes of a minimum in the aforementioned graph is 0; the gradient of a transition state in both directions is 0 too. A transition state would be a saddle point on this surface so they could be distinguished by calculating the second partial derivative: the second partial derivative of a transition state along one axis would be negative, and along the other axis it would be positive; whereas this value would be positive along both axes for a minimum.
(Overall, good discussion. However, the second partial derivatives at the TS are not calculated along either axes, but rather along the reaction coordinate and its orthogonal axis. E.g. in the case of HHH, this is turned by 45° with respect to the axes. Fjs113 (talk) 21:11, 10 May 2018 (BST))
Transition state
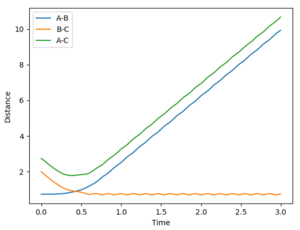
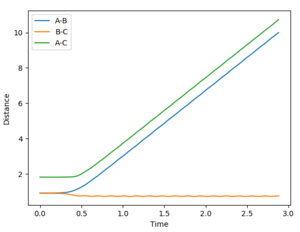
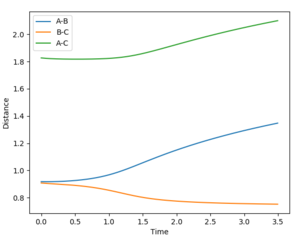
Estimate transition state distance, rts, = 0.908 Å. The internuclear distance vs time plot shows that there is no oscillation in the lines representing the AB and BC distances: at the TS, there is no internuclear vibration as AB and BC are at the dissociation limit.
Changing the calculation type
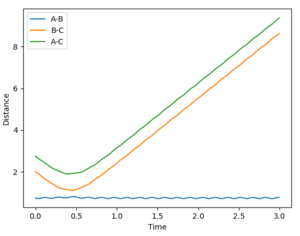
- Dynamics: since the atoms have a momentum realistically, this method shows the molecular vibrations.
- MEP: since the velocity resets to 0 after each step, the H2 molecule formed shows no vibrations, indicated by the smooth A-B curve. Reversing the dynamics calculation doesn't reverse the reaction.
| Dynamics calculation | MEP calculation | Dynamics calculation, reversed trajectory |
|---|---|---|
|
|

|
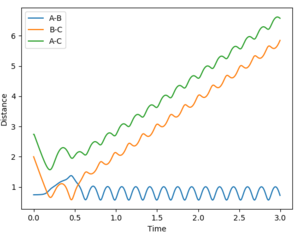
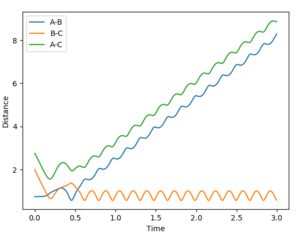
Reactive and unreactive trajectories
(Missing total energy data. Also, a contour plot would've illustrated the results from the different initial conditions better. Lastly, a more in-depth discussion of each case, mentioning how the different momenta influence different types of energies, would've shown a deeper understanding. Fjs113 (talk) 21:11, 10 May 2018 (BST))
Transition state theory
Transition State Theory assumes that there is a "quasi-equilibrium" between the reactants and activated complex and not between the latter and the products.[1] It also assumes the if the reactants do not collide with an energy above the activation energy, the reaction will not occur. Activation energy predictions using this theory may slightly differ from experimental values for reactions with a low activation energy because it doesn't take quantum mechanics into account: quantum tunneling could lower the activation energy, so the reactants won't have to collide with as much force.
(Yes, but this is only half the story: Barrier recrossing (as seen in cases 4&5) is not taken into account by TST. Fjs113 (talk) 21:11, 10 May 2018 (BST))
Exercise 2: H2 + F
Classifying reactions
The trough corresponding to the H-F bond is deeper than the one for the H-H bond, meaning that the H-F bond is stronger than the H-H bond. In other words, the potential energy of the reactants is less negative than that of the products. Therefore, the F + H2 --> HF + H reaction is exothermic and the reverse reaction is endothermic.
(This should've been supported by a figure. Fjs113 (talk) 21:11, 10 May 2018 (BST))
Transition state
Using Hammond's postulate, the transition state should be early as F is the most electronegative atom, i.e. the H-H bond distance at the transition state should be close to the bond distance of a free H2 molecule (0.74 A). The approximate calculated H-H bond length at the TS was 0.745 A and a H-F bond length of 1.8107 Å
(Some more details of the derivation are needed here. Fjs113 (talk) 21:11, 10 May 2018 (BST))
Activation energy
(Parameters: MEP, F-H = 1.7507, H-H = 0.745, 10000 steps). TS = -104, H2 = -103.869 and HF = -135 kcal mol-1
- Activation energy of forward reaction = +0.131 kcal mol-1
- Activation energy of reverse reaction = +31.131 kcal mol-1.
(Again, a picture or two would've supported these rather bleak and bland numbers. Simply stating the values and some parameters is not enough: Why those particular parameters? Fjs113 (talk) 21:11, 10 May 2018 (BST))
Reaction dynamics of F + H2
The pHF value reaches high values in the program, but since energy has to be conserved, this vibrational energy is released as heat through internal conversion, thus allowing HF to go to its ground vibrational state. You could confirm this by measuring the enthalpy change using calorimetry or using a thermometer.
With the parameters of rHH = 0.74, rFH = 2.3 and pFH = -0.5, the following was observed: a pHH value of -2.8 led to a reaction, but deviation of 0.5 or more away from this value led to barrier crossing and the reaction didn't occur, despite the high amount of energy going into the H-H vibration.
| pHH = -2.8 | pHH = -2.75 | pHH = -2.85 |
|---|---|---|

|

|

|
Using the values of pHF = -0.8 and pHH = 0.1,
Reaction dynamics of H + HF
For rHF = 0.935 and rHH = 2.3, it was difficult to yield a reaction with a low vibrational energy and a high translational energy; a very high translational energy energy was needed for a reaction to occur, whereas a relatively lower vibrational energy was needed to the same effect. This concurs with Polanyi's rules of a high vibrational energy being more effective in allowing a reaction to progress than translational energy for a reaction with a high activation energy.[2]
An exothermic reaction leads to an early transition state and the converse is true for an endothermic reaction. According to Polanyi's rules, the translational energy doesn't have much of an effect in dictating whether a reaction occurs if it has an early transition state, unlike the vibrational energy (vice versa for a late transition state. Therefore, the program concurs with the predictions of these rules, and therefore for the forward reaction, the translational energy would be the more dominant factor.[3]
| pHF = -5
pHH = -10 |
pHF = -15
pHH = -8 |
|---|---|

|

|
- ↑ IUPAC Gold Book - transition state theory. [online] Goldbook.iupac.org. Available at: http://goldbook.iupac.org/html/T/T06470.html [Accessed 4 May 2018].
- ↑ Zhang, Z., Zhou, Y., Zhang, D., Czakó, G. and Bowman, J. (2012). Theoretical Study of the Validity of the Polanyi Rules for the Late-Barrier Cl + CHD3 Reaction. The Journal of Physical Chemistry Letters, 3(23), pp.3416-3419.
- ↑ Zhang, W., Kawamata, H. and Liu, K. (2009). CH Stretching Excitation in the Early Barrier F + CHD3 Reaction Inhibits CH Bond Cleavage. Science, 325(5938), pp.303-306.