MRD:sk4915
Exercise 1: H + H2 system
What value does the total gradient of the potential energy surface have at a minimum and at a transition structure? Briefly explain how minima and transition structures can be distinguished using the curvature of the potential energy surface.
The gradient at both the minima and at transition structures is zero. The minima and transition structures can be distinguished as transition structures only occur at maxima.

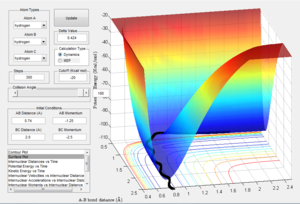
Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” screenshot for a relevant trajectory.
Transition state position = 0.9077425 A. It can be seen that the internuclear distance doesn't appear to change when the system is started with r1 = r2 = 0.9077425 and with p1 = p2 = 0. Furthermore, a kinetic energy-time graph shows the small vibration about the bond equilibrium with a total kinetic energy of 1.8e-15 J.
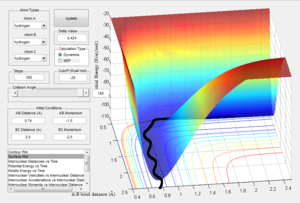
Comment on how the mep and the trajectory you just calculated differ.
With the starting conditions as r1 = rts + 0.01, r2 = rts and p1 = p2 = 0, the same calculation to show the surface plot for the reaction was run with two different methods. When run in mep the trajectory follows the valley floor very closely and doesn't oscillate unlike the dynamics trajectory which does oscillate. When the r1 and r1 are switched the reaction proceed in the opposite direction.
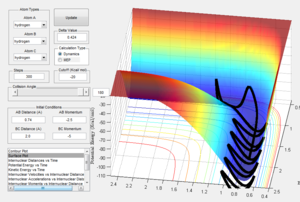
Table to show how initial momentum affects trajectories, making them reactive or unreactive, with initial positions r1 = 0.74 and r2 = 2.0:
State what are the main assumptions of Transition State Theory. Given the results you have obtained, how will Transition State Theory predictions for reaction rate values compare with experimental values?
Transition state theory assumes that nuclei behave according to classical physics and ignores quantum effect such as tunneling which can have significant effects for reactions with low activation energies. This could mean that reaction rates predicted with transition state theory will be lower than experimental values as tunneling would increase the number of particles that cross the potential barrier. Further, transition state theory assumes that each step in the reaction is long lived enough to reach a Boltzmann distribution of energies. Experimentally this may not always be the case so some steps will have different energies than the theory predicts, changing the speed at which products are formed and also possibly changing which products are formed.
Exercise 2: F - H - H system
Classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved?
F + H2 is an exothermic reaction and H + HF is endothermic. This is because more energy is released when an F-H bond is formed than is needed to break an H-H indicating that the F-H bond is the much stronger bond type.
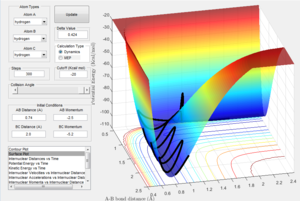
Locate the approximate position of the transition state.
For the reaction F + H2, according to the Hammond postulate the transition state will more closely resemble the reactants than the products as the reaction is exothermic. As a result it can be seen that the transition state occurs at approximately r1 = 0.80, r2 = 0.75. Additionally, by looking at the potential energy surface it can be determined that the activation energy for the F + H2 reaction is 0.2 kcalmol-1 and 30.2 kcalmol-1 for FH + H reaction.
In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. How could this be confirmed experimentally?
The reaction F + H2 is exothermic which means that some of the kinetic energy used to cause the reaction is stored in the newly formed F-H bond, which is much stronger than the previous H-H bond. To confirm experimentally that this is where the energy is going, the reverse endothermic reaction could be carried out where the amount energy that must be added is equal to the energy 'lost' during the exothermic reaction.
Discuss how the distribution of energy between different modes (translation and vibration) affect the efficiency of the reaction, and how this is influenced by the position of the transition state.
For reactions with early transition states the reaction efficiency will be greater when there is initially more translational energy than vibrational energy as the reactants quite closely resemble the transition state and so can rearrange to form the transition state with little additional energy. Therefore, the close proximity of the reactants is more valued more highly and more collisions will occur when the translational energy is higher. The inverse is true for reactions with late transition states.