MRD:saramalek
H + H2 system
Gradient of the potential energy surface
QUESTION ONE
dV/dr = 0 (where r= r1 and r2 i.e. both partial derivatives = 0) at minimum points and transition structures (saddle points), as a zero gradient represents turning points. A minimum point and saddle point can be distinguished by taking the second partial derivative test; the Hessian determinant ,D, is calculated. If:
- D<0 : Saddle point.
- D>0 AND the second partial derivative is >0: Minimum point.
Locating the transition state
QUESTION TWO
rts=r1 = r2 = 0.908 Å
| Internuclear Distance VS Time Graph | Analysis |
|---|---|
 |
The graph shows two approximately linear lines i.e. there is no/very little oscillation in energy, therefore it is a saddle point. |
Mm10114 (talk) 20:43, 31 May 2018 (BST) The graph looks good. However, you should've explained what steps did you take to find the transition state.
Reaction path
QUESTION THREE
Mm10114 (talk) 20:44, 31 May 2018 (BST) Very good observations. But, why is MEP following the lowest energy path? Some cause->effect explanation would be still necessary.
Reactive and unreactive trajectories
QUESTION FOUR
Transition State Theory
QUESTION FIVE
- Assumptions:
- Classical mechanics
- The transition state will be in (quasi) equilibrium with the reactants
- The transition state converts to products every single time
The third prediction means that the theory doesn't account for barrier recrossing and reversion of TS to reactants, which can be seen in the experimental results from question 4. Therefore, the theory overestimates the rate of reaction as it assumes that the transition state always breaks down to form products, i.e. a slower experimental value observed.
However, the first assumption also doesn't apply in all situations. The H atom could tunnel through the activation barrier, a process not accounted for by classical mechanics but by quantum mechanics, as it means that the H atom has wave-like properties (wave-particle duality). Tunneling depends on barrier height, thickness and the mass of the particle, therefore for a (relatively) light H atom tunneling is viable and would thus increase the rate of reactions - in this case the theory underestimates the rate of reaction. For heavier reactants, tunneling would probably be negligible as tunneling wouldn't be favourable, and the classic approach of transition state theory dominates.
Therefore, in the H H2 system, the experimental rate could be higher than predicted due to tunneling, or lower than predicted due to barrier recrossing; in this case it would seem appropriate that the experimental rate is LESS than the predicted rate as the barrier recrossing effects are more predominant than tunneling due to the requirements for tunneling.
Mm10114 (talk) 20:45, 31 May 2018 (BST) Very good discussion. However, where are these assumptions of TST taken from? You're missing the most important part of the answer - referencing the source.
F-H-H System
PES Inspection
QUESTION SIX
- The H-F bond is stronger than the H2 bond due to the large electronegativity difference between H and F, which polarises the bond introducing strong ionic character. Therefore, the 'F + H2' reaction will be exothermic (as it includes making a strong bond and breaking a relatively weak one) and the 'H + HF' system will be endothermic (as the strong H-F bond needs to be broken).
QUESTION SEVEN
- As 'F + H2' (the reactants) is higher in energy, it is closer in energy to the transition state, according to Hammond's postulate - i.e. in an exothermic reaction the transition state will resemble the reactants (energetically and structurally). The surface plot below shows the position of the transition state (black dot) - the energy of the transition state is -103.752 kcalmol-1.
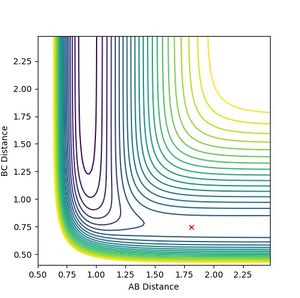
QUESTION EIGHT
- The internuclear distance vs time graph below shows the F-H and H-H bond lengths at the transition state; F-H=A-B= 1.810 Å and H-H=BC= 0.745 Å. The plot shows the transition state because the bonds do not oscillate with time i.e. minimum energy.

- EA= E(TS) - E(Reactants)
- Performed an MEP with 500000 steps, with the F-H = 1.9 Å (slightly distorted from TS) and H-H= 0.74 Å. When the reactants are F and H2:
- EA = -103.752 - - 104.008 = +0.256 kcalmol-1 EXO
- Performed an MEP with 500000 steps, with the F-H = 1.8 Å and H-H= 0.8 Å (slightly distorted from TS). When the reactants are H and H-F:
- EA = -103.752 - - 103.877 = -0.125 kcalmol-1 ENDO
- Performed an MEP with 500000 steps, with the F-H = 1.9 Å (slightly distorted from TS) and H-H= 0.74 Å. When the reactants are F and H2:
Mm10114 (talk) 20:50, 31 May 2018 (BST) Are you sure this is the correct value for when the reactants are H and H-F? Earlier you have discussed that the H-F bond is much stronger, so breaking it will require energy, thus endothermic reaction. Your value of -0.125 kcal/mol does indicate endothermic reaction, but it does not indicate this is a strong bond to break. It should've alert you, especially that it is half of the magnitude of that you've provided for breaking of H-H bond (although opposite sign). If you would revisit the 3D surface plot, and observe the products/reactants valleys (and look on the Z axis that gives you potential energy) you would see that the difference between the two is much much higher.
Reaction dynamics
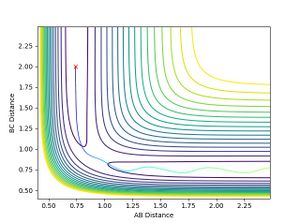
Reactive trajectory of F and H-H
QUESTION NINE
- The reaction of F with H2 is an exothermic reaction therefore energy is released. To conserve energy, this energy is stored in the new F-H molecule as vibrational energy, i.e. it vibrates. This could be confirmed experimentally by measuring IR spectrum of and H-F; the stretching of H-H is IR inactive because it is symmetric.
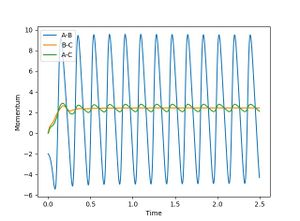
- The momentum vs time plot shows that H-H (BC) has constant momentum after the transition state whereas F-H (AB) has an oscillating momentum - this shows that the final H-F molecule is vibrating.
Polanyi Rules
QUESTION TEN
Polanyi rules state that vibrational energy activates a late transition state more efficiently than translational energy, and the opposite is true for an early transition states. (Guidelines but not rules set in stone.)
Mm10114 (talk) 20:50, 31 May 2018 (BST) Reference?
F + H2
- An exothermic reaction with an early transition state.
- F-H momentum = translational energy (fixed at -0.5)
- H-H momentum = vibrational energy
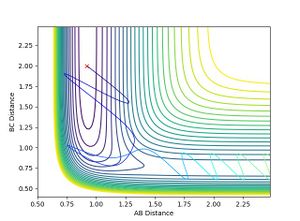
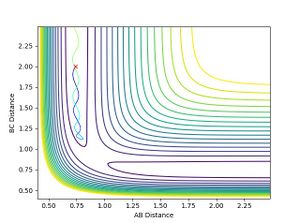
- When H-H momentum = 0.3, i.e. vibrational energy is less than translational, the trajectory is reactive.
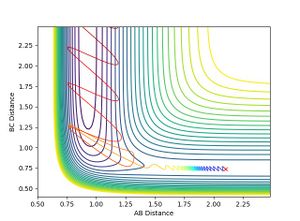
- When H-H momentum = 3, i.e. vibrational energy is more than translational, the trajectory is unreactive.
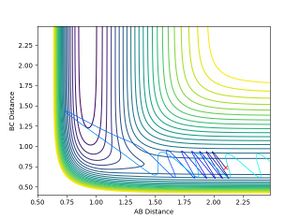
- This shows that for an early transition state, the translational energy has a greater effect on the efficiency of reaction. i.e. the translational energy activates the TS more efficiently than vibrational energy leading to a reactive trajectory, agreeing with Polanyi rules.
H + HF
- An endothermic reaction with a late transition state.
- F-H momentum = vibrational energy
- H-H momentum = translational energy
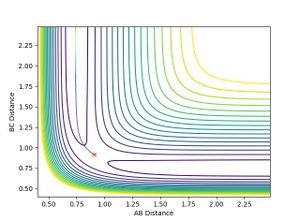
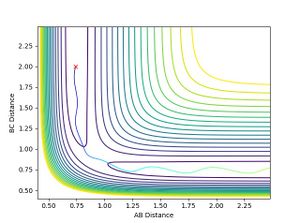
- When H-F momentum = 0.1 (LOW VIBRATIONAL ENERGY) and H-H momentum = -5 (HIGH TRANSLATIONAL ENERGY), the trajectory is unreactive.
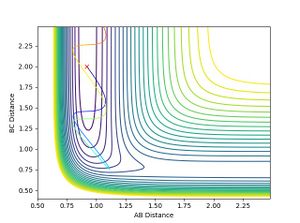
- No matter how much the H-H momentum (translational energy) is increased, the trajectory will recross the barrier and return to reactants. This is because the vibrational energy, F-H momentum, is insufficient for the reaction to occur, therefore it follows Polanyi rules as it agrees that vibrational energy is more efficient for crossing late transition states. This is shown below:
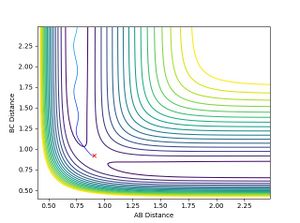
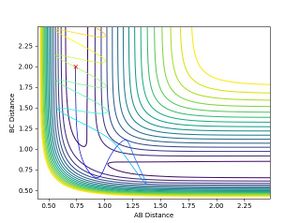
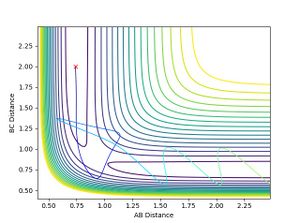
- When H-F momentum = 7.5 (HIGH VIBRATIONAL ENERGY) and H-H momentum = -1.4 (LOW TRANSLATIONAL ENERGY), the trajectory is reactive.