MRD:saladhorse
Molecular Reaction Dynamics: Application to triatomic systems
Application to the collision of H radical to H2 molecule
Introduction
The reaction investigated in this section is a simple triatomic reaction involving diatomic hydrogen and hydrogen radical. The hydrogen are labelled as A, B and C.
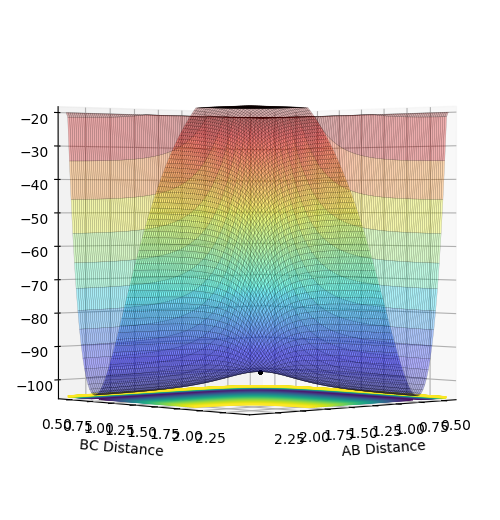
Significance of gradients in surface plots
The gradient of the minimum path potential energy is always equals to zero and is what the trajectory of the minimum energy path (MEP) follows. The minima is the lowest energy possible along the minimum path potential energy and is towards the entry and exit paths (the products/reactants) and in this case the same. The transition state is the maximum of the minimum energy path which is the saddle point of the potential energy. It satisfies the expression:
dV/dr1=dV/dr2=0
Jas213 (talk) 14:43, 28 May 2018 (BST) What about the second derivative?
Determination of the transition state distances
When the initial condition is at the transition state distance and has 0 movement, the system remains at rest and there is no fluctuation of the internuclear distances. From calculation, the estimate transition state distances between the hydrogen atoms is 0.907742 angstroms. When the initial conditions are set as such, the internuclear distances does not fluctuate.
Changing the calculation types and parameters
Minimum energy path (MEP)
Because the transition state is the local maximum, nudging the setup either way will force it to collapse into the reactant/product. By nudging the setup very slightly to one side and changing the calculation type to MEP, the MEP reaction pathway can be determined as the trajectory follows a very slow motion.
The difference between MEP and dynamic type calculations
In minimum energy path (MEP), the atoms move a lot slower than the dynamic calculation type and does not show oscillation motion. The final A-C distance value for MEP approaches 2.0 units however the dynamic trajectory is approximately 10.0 units in the same amount of time (2.5 time units), this is due to MEP calculation type modelling infinitely slow motion down the MEP.
Changing the parameters
Given that all three atoms are initially at rest, when r1=0.918=rts+0.01, and r2=0.908=rts, the final values when using dynamic calculation type (t=2.5 time units) are:
r1=9.03 and r2=0.75
p1=2.48 (constant) and p2=1.22 (on average)
If we were to to swap the initial values of r1 and r2, the calculated values will be the same but swapped such that r1 becomes r2 and vice versa.
By setting up the initial positions as the calculated final positions from above but with the negative value of the calculated momentum, essentially the atoms will approach each other and achieve the transition state at the same amount of time as the motions are played back. This is the inversion of momentum procedure which follows a time symmetrical pathway.
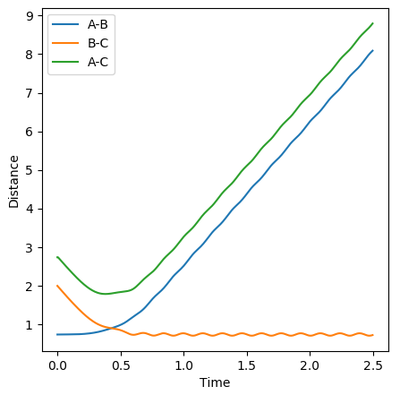
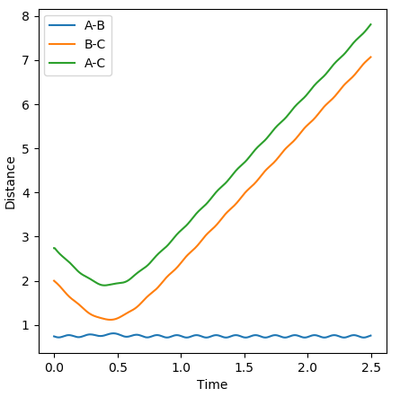
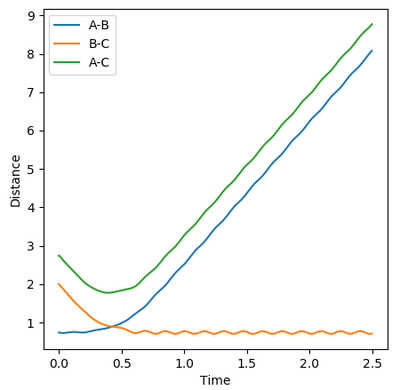
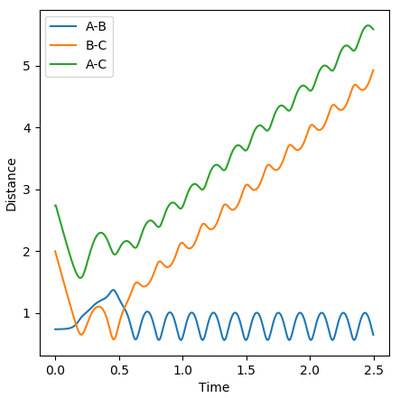
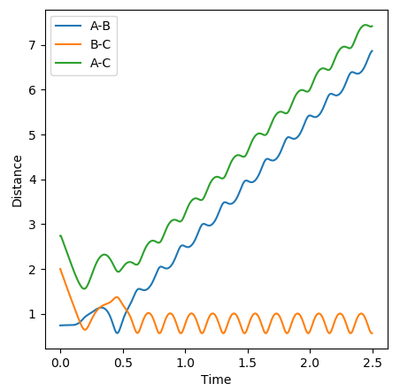
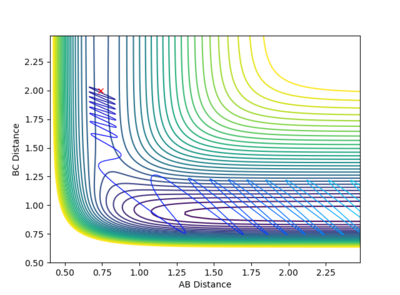
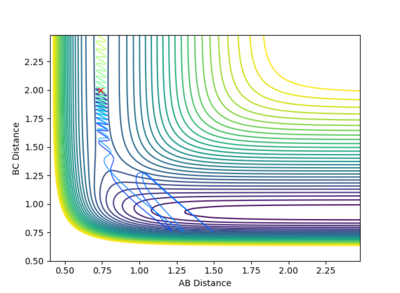
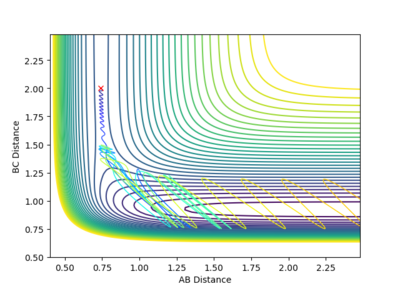
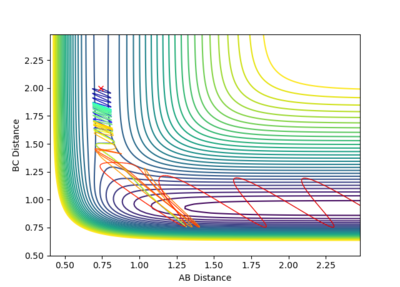
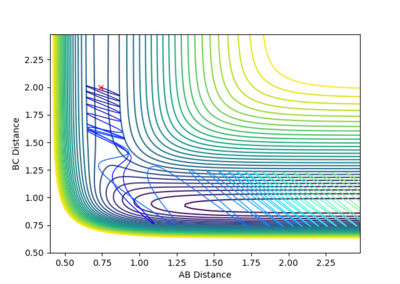
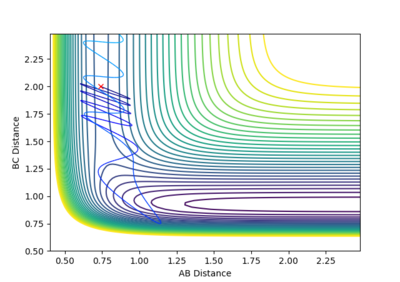
Reactive and Unreactive trajectories
Collisions need to have enough kinetic energy to be able to go over the activation energy barrier and form the product and so one can assume that beyond the threshold momentum, the reaction will be successful as it has enough kinetic energy, but this is not always the case. The table below shows the total energy of the system and determine if the reaction is successful or not.
Despite the fourth calculation having a higher total energy than the first calculation on the table above, the reaction is unsuccessful, demonstrating that higher kinetic energy does not always mean the reaction will be successful; this can be attributed to barrier recrossing. For all graphs the distance between atom B and C is the new bond that is going to be formed. A successful bond is made when the distance oscillates between a value around 0.7 angstroms.
Jas213 (talk) 14:46, 28 May 2018 (BST) First sentence is a good start to an overall conclusion of what you learned from the table.
Transition state theory
Transition state theory (TST) assumes that chemical reactions, whether reversible or irreversible, undergoes a quasi-equilibrium with the transition state and it assumes that there is no quantum tunneling effects; if the system does not have enough energy to cross the activation energy then the reaction will not occur. TST also assumes that the reaction will proceed at the lowest possible energy and once pass the energy barrier, will not fall recross the barrier back to the reactant. Because of this, it fails to predict barrier recrossing effect as the transition state formed at higher temperatures (the particles involved have high momentum) and the transition state can be a lot higher than the lowest possible energy. The kinetics predicted by TST at higher temperature is therefore going to be higher than experimental values as it does not take into account barrier recrossing.
Jas213 (talk) 14:46, 28 May 2018 (BST) Where are your references for this?
Application on F-H-H System
Introduction
The reaction investigated in this section is the reaction of fluorine radical to diatomic hydrogen to form hydrogen fluoride and hydrogen radical. The reaction will be treated as "reversible". In the calculation, Atom A and B are hydrogen atoms and atom C is the fluorine radical.
Potential Energy Surface Inspection
Reaction Energetics
In the reaction F + H2 ⇌ HF + H, the forward reaction is exothermic and the reversed reaction is therefore endothermic; this is due to the formation of HF bond which is stronger (569 kj/mol) compared to HH bond (435 kj/mol).
Jas213 (talk) 14:47, 28 May 2018 (BST) kJ/mol
The transition state bond lengths of the system is determined to be approximately these values:
HH=0.745 angstroms
HF=1.811 angstroms

The calculated activation energy for the forward reaction, F + H2, is 0.268 kcal/mol whilst the reversed reaction,HF + H, is 30.247 kcal/mol rendering this reaction essentially irreversible at room temperature.
Jas213 (talk) 14:48, 28 May 2018 (BST) Correct, but how did you obtain these values? Where are your MEP plots for this?
Reaction Dynamics
Conservation of Energy
Given that A and B are hydrogen atoms and C is a fluorine atom in a linear collision trajectory, setting AB distance to 0.75 and BC distance to 3 angstroms and the BC momentum of -1.2 units, the reaction is successful and because of the strong attraction between B and C, this causes atom A to be ejected at a relatively high speed and the vibration of H-F bond to be quite vigorous. Collectively, the vibration and translation is observed in the form of heat. By using a calorimeter to measure the temperature difference after the reaction, the thermal energy observed can be compared to the calculated energy values. The energy released as vibrational mode could be measured by measuring the infrared radiation emission at the H-F stretch region.
Polanyi's rules
Setting up the calculation to be towards the reactants of the forward reaction (F radical and diatomic hydrogen), the H-H bond length is set as 0.74 and F-H distance to be 2.0 angstroms. The initial translational momentum is set to be -0.5 and the vibrational momenta for H2 will be -3, -2, -1, 0, 1, 2 and 3 units:
The higher the absolute value of PHH, the higher the total energy in the system, all with way more energy than the activation energy and some reactions are successful while some are not due to the unpredictability of barrier recrossing. Higher vibrational energy and therefore the total energy does not always determine the success of this particular reaction which contradicts with the TST.
Changing the initial value slightly so that PHH is -0.1 and the momentum of F radical is -0.8 gives this result:

Despite the total energy of the system being significantly lower than most of the trajectory calculations above, the reaction is successful demonstrating that the translational energy is more efficient than the vibrational energy in this case.
On the flip side, setting up the calculation so that the reaction proceeds backwards (from H + HF to H2 + F), the initial condition to a successful reaction would be this:

Jas213 (talk) 14:50, 28 May 2018 (BST) Good reasoning, shows clear understanding of the material studied and the proportions of vibrational and translational energy.
The initial energy in this calculation is relatively low but yields a successful reaction. When looking at how the energy is distributed, the vibrational mode is much higher than the translational mode, swapping these values such that the total energy of the system is the same will result in an unsuccessful reaction.
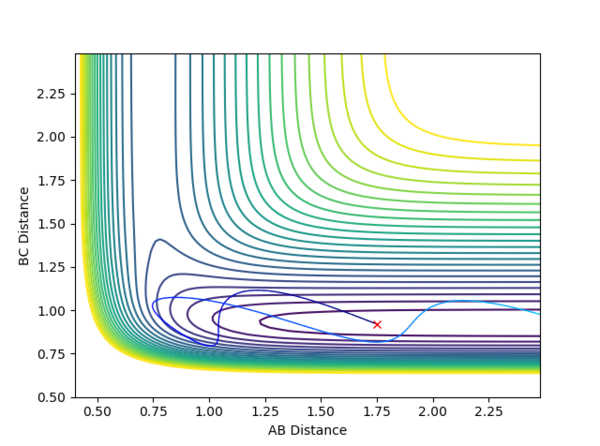
In the case of the reverse reaction, a high translational fraction of the same total energy is not as efficient as high vibrational fraction; at a reasonably low energy just above needed for activation, the translation motion just gets repelled back without crossing through the transition state where as the high vibration allows the system to cross through the exit channel. By applying the inversion of momentum procedure, if high vibration low translation system is successful for the reverse reaction, the forward reaction would be opposite; high translation low vibration tend to be more successful.
To conclude, this demonstrates Polanyi's empirical rule which states that for a reaction with proximal transition state, the momentum is more deterministic than vibration for the reactivity and for reactions with late transition state, the opposite applies. Since the transition state is a lot closer to F + H2, the forward reaction will be more determined by the momentum and the reverse reaction will depend more on the vibrational energy.
Jas213 (talk) 14:53, 28 May 2018 (BST) You mean translational energy when you say momentum? Both p1 and p2 are momenta. Correct conclusion, however sadly no references given for Polanyi's rules.