MRD:qx5117
Molecular Reaction Dynamics: Applications to Triatomic systems
EXERCISE 1: H + H2 system
Q1:On a potential energy surface diagram, how is the transition state mathematically defined? How can the transition state be identified, and how can it be distinguished from a local minimum of the potential energy surface?
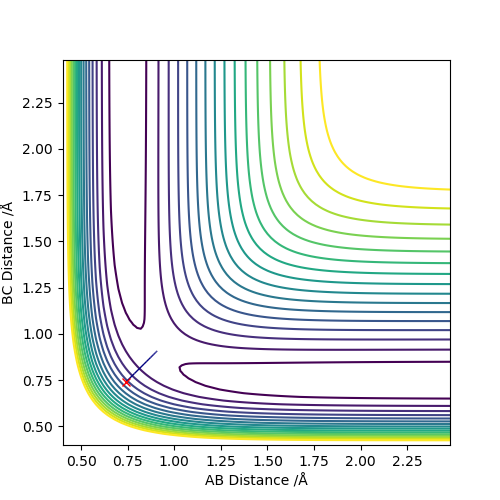
To obtain the transition state, we import a new sets of coordinates q1 & q2. Their directions are shown as above in the diagram, where q1 is describing where the atom A and atom C approaching and aparting away from atom B simultaneously and q2 is actually the reaction coordinate where atom C approaching B as atom A moving apart from B.
To find out the transition state mathematically,
Find q1 and q2 when ∂V(q1)/∂q1=0 & ∂V(q2)/∂q2=0 and ∂V2(q1)/∂q12 > 0 & ∂V2(q2)/∂q22 < 0.
For a local minimum,
First, any coordinates either (r1, r2) or (q1, q2) have Vx = Vy= 0 it is a stationary point. If it is a local minimum, it also fulfil that, Vxx * Vyy - (Vxy)2 >0 & Vxx > 0.
Good Mak214 (talk) 10:29, 23 May 2019 (BST)
Q2:Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” plot for a relevant trajectory.
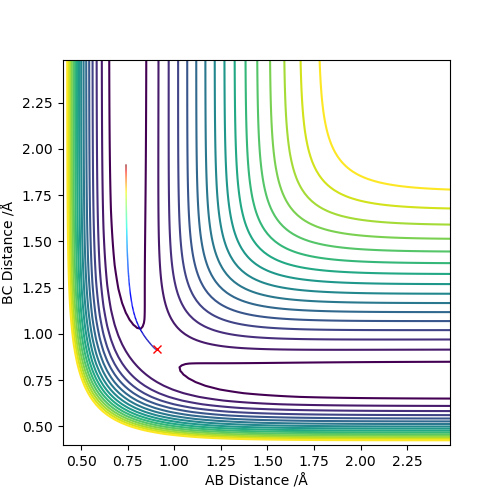
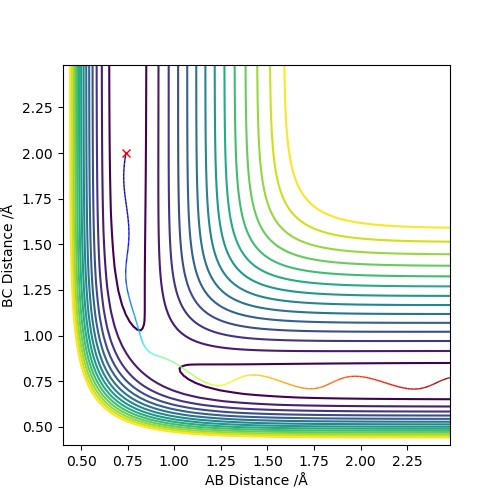
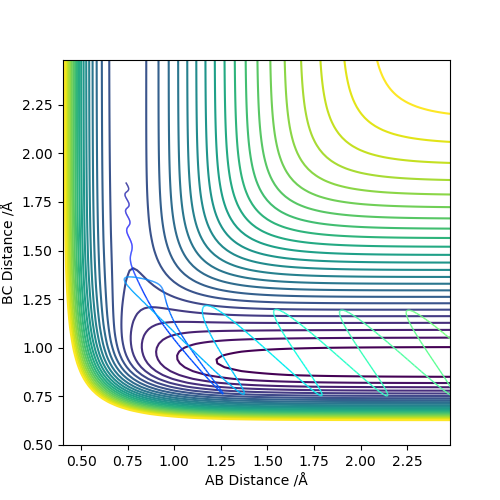
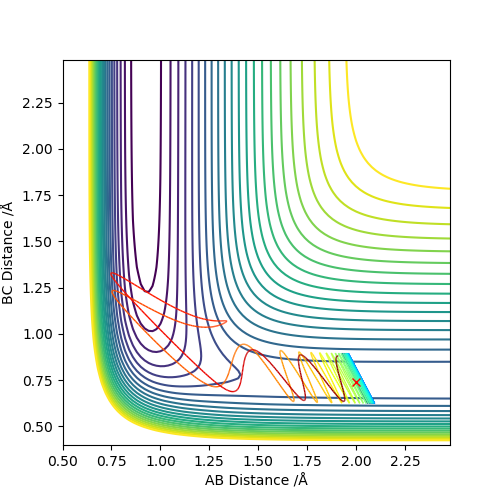
Set the initial r1=r2=0.74 to obatain a contour plot of the distance AB and BC. Zoom in to locate the coordiante of the blue line across the graph and the coordiante of the top right end of the line is the point where this separation of AB and BC gives the minimum potential energy.
Using MEP method with same parameter, the stright line part of BC curve also shows the rts of the transition state position. rts = 0.9068 Å
Good. Mak214 (talk) 10:30, 23 May 2019 (BST)
Q3: Comment on how the mep and the trajectory you just calculated differ.
Set the r1 = 0.916, r2 = 0.906 and p1 = p2 = 0.0.
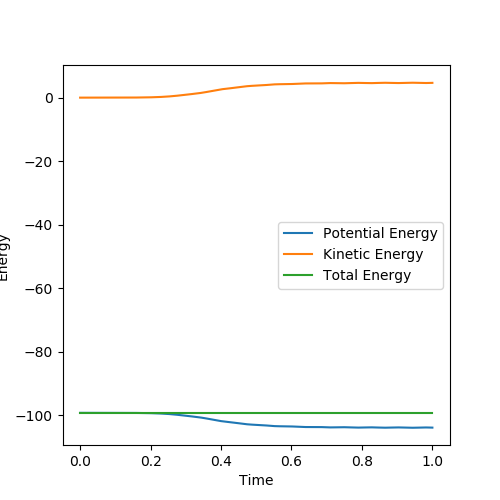
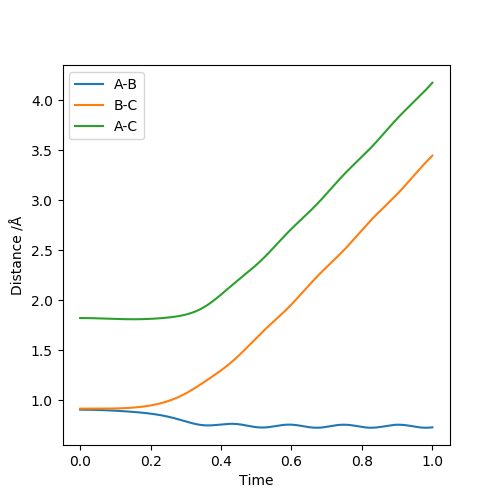
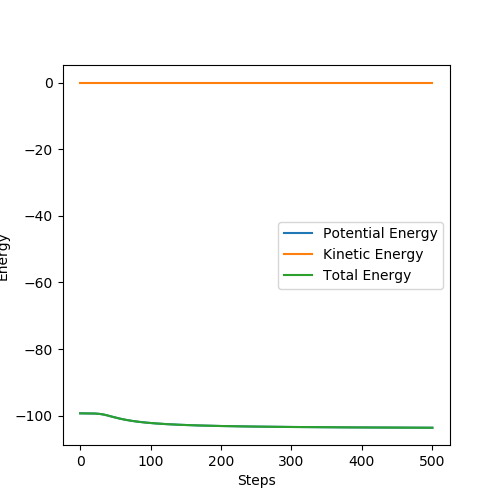
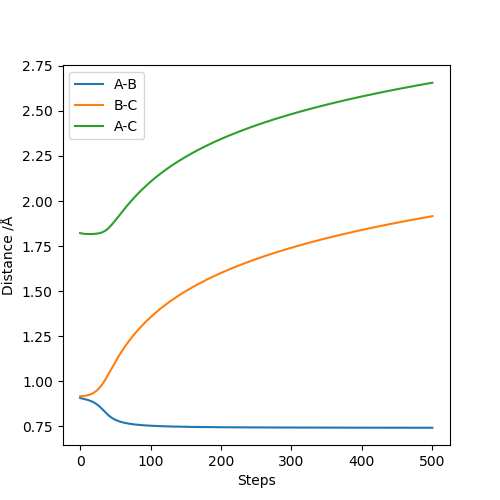
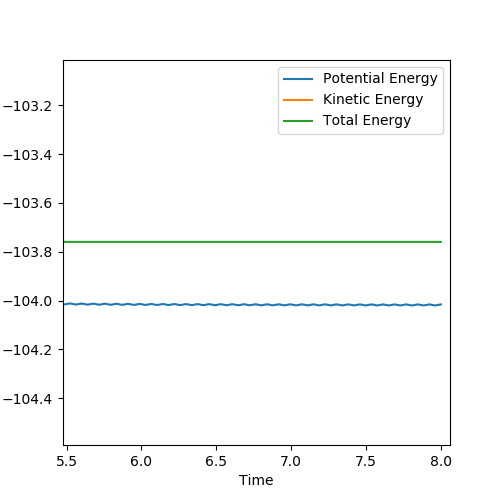
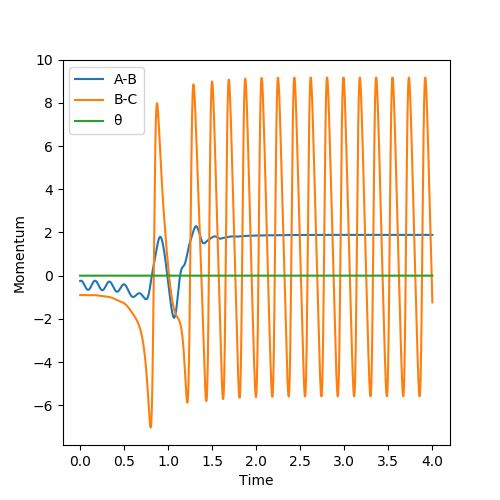
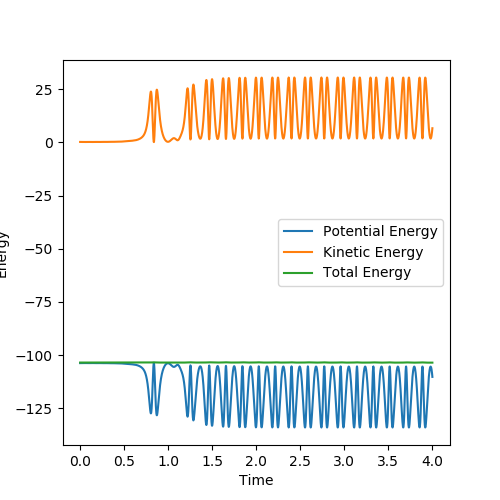
Below is the plot by using Dynamics method. As it can be seem that the potential energy decrease as the kinetic energy increase by the equal amount due to the product is formed as the other atom move apart from the product. The total energy is conserved. The vibration in the contour plot and the internuclear distance vs time plots showed that there is still bond vibration after the product formed which is due to the nature of vibration of the atoms in the molecules.
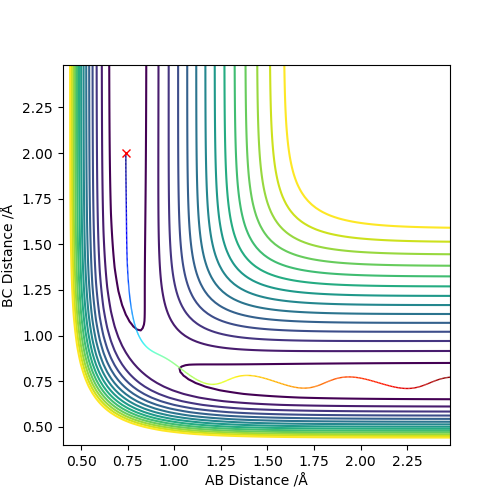
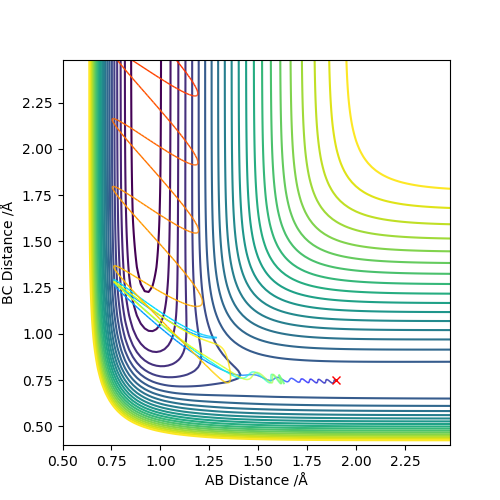
Below is the plot by using MEP method. The reaction path is broken at the BC distance at around 1.9. The kinetic energy is zero along the whole reaction pathway and there is decrease in potential energy so the there is a decrease in the total energy. The distance vs time graph showed that the there is no bond vibration in the product which mean the momentum and vibrations are ignored in this MEP method.
OK. Think about why these two plots were different, i.e. what did the calculation methods do differently? Mak214 (talk) 10:31, 23 May 2019 (BST)
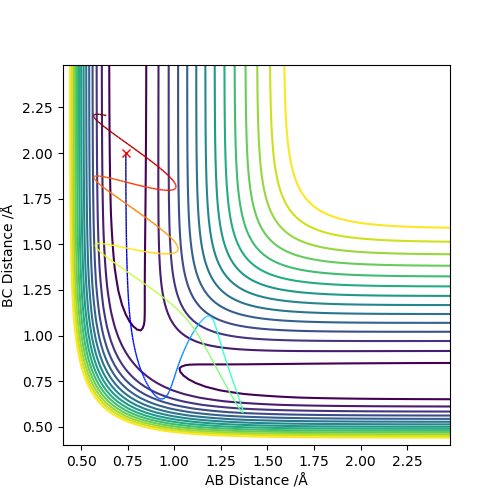
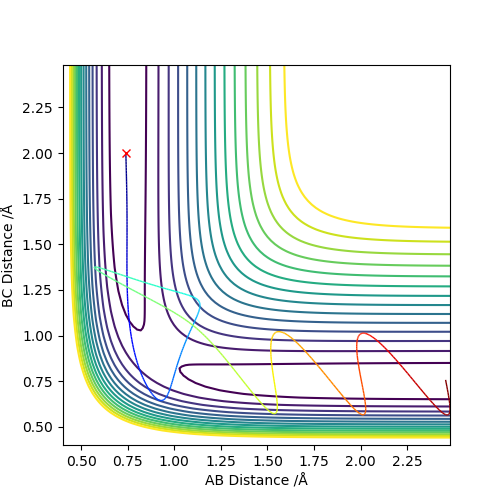
Q4: Complete the table above by adding the total energy, whether the trajectory is reactive or unreactive, and provide a plot of the trajectory and a small description for what happens along the trajectory. What can you conclude from the table?
The initial positions are set as r1 = 0.74 and r2 = 2.0,trajectories are run with the following momenta combination
In conclusion:
Enough kinetic energy is required for the reaction to carry on. If there is not enough energy, the atom cannot overcome the repulsion boundary. High energy applied on the reactants may lead to a unreactive reaction. Since the product can be formed in the first place but due to high energy, it is very unstable and reactive. The new product will bouncing in the repulsive potential energy region and eventually breaks down to go backward to reform the reactants. In some cases, high energy may still give out the product, there is no prediction on whether the new formed product will go which direction of the reaction.
OK. What about vibrational energy? Mak214 (talk) 10:33, 23 May 2019 (BST)
Q5: State what are the main assumptions of Transition State Theory. Given the results you have obtained, how will Transition State Theory predictions for reaction rate values compare with experimental values?
Assumptions1:
1. Molecular systems that have crossed the transition state in the direction of products cannot turn around and reform reactants.
2. In the transition state, motion along the reaction coordinate may be separated from the other motions and treated classically as translation.
3. Even in the absence of an equilibrium between reactant and product molecules, the transition states that are becoming products are distributed among their states according to the Maxwell-Boltzmann laws. The meaning is not quite clear. I think the assumption you are referring to is that the energy of the particles in the system has a Boltzmann distribution. Mak214 (talk) 10:37, 23 May 2019 (BST)
Results:
1. The first assumption is not supported by the experimental data. From the situation 4 in the Q4, there is chance that the new formed product to break down back to the reactants due to the large momentum in the atoms. In some cases, it will reform reactants while some will not. There is no further information allowed to predict the result if larger amount of momentum is applied.
2. There is not enough information to study the motions during the transition state. It only shows a point in the contour plot.
3. Not enough information to support that the amount of transition states becoming products obeys the Boltzmann law.
Good. Mak214 (talk) 10:37, 23 May 2019 (BST)
EXERCISE 2: F - H - H system
By inspecting the potential energy surfaces, classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved?
For reaction F + H2, the reaction is exothermic. The HF bond formed is more stable/higher bond energy than the H2 bond before.
For reaction HF + H, the reaction is endothermic, more energy is required to break the HF bond and the energy gained of HH bond(432 kJ/mol) is smaller than the energy loss of the HF bond (565 kJ/mol).
 Good. Mak214 (talk) 10:46, 23 May 2019 (BST)
Good. Mak214 (talk) 10:46, 23 May 2019 (BST)
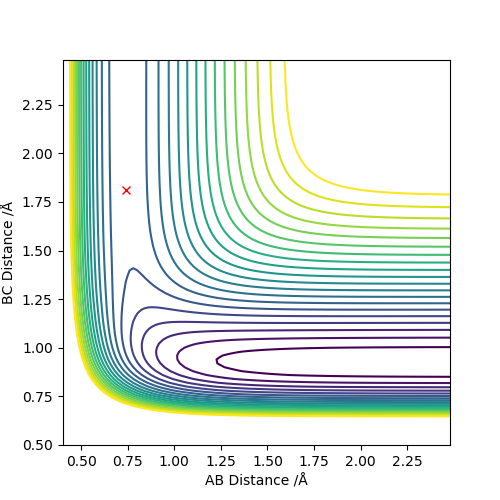
Q6: Locate the approximate position of the transition state.
for reaction of F + H2 & HF + H: rHH = 0.74, rHF =1.81 the contour plot only showed a point an the internuclear distance vs time plot is consist of three straight lines.
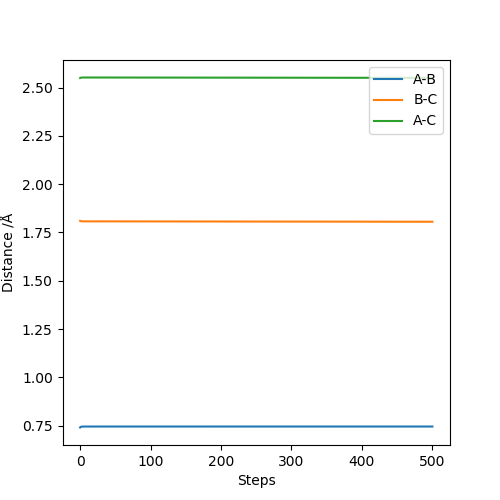 Good. Mak214 (talk) 10:46, 23 May 2019 (BST)
Good. Mak214 (talk) 10:46, 23 May 2019 (BST)
Q7: Report the activation energy for both reactions.
The activation energy of the H2 + F is the energy difference between the total energy before reaction and after the reaction (the difference between two straight lines) E= 0.30 kcal/mol
The activation energy of the HF + H, E= 30.2 kcal/mol
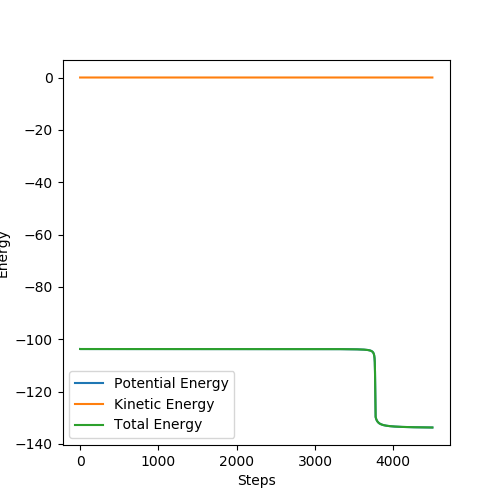 Good. Mak214 (talk) 10:46, 23 May 2019 (BST)
Good. Mak214 (talk) 10:46, 23 May 2019 (BST)
Q8: In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. Explain how this could be confirmed experimentally.
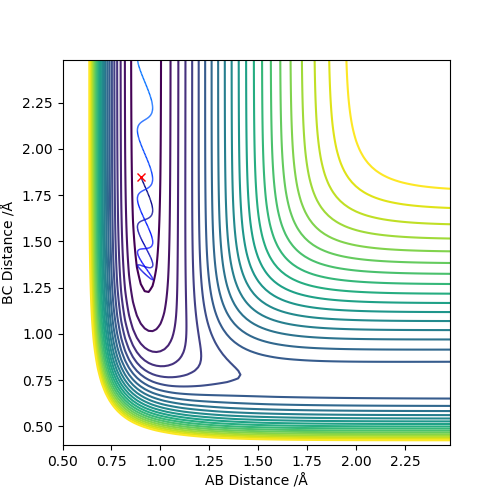
The initial condition is set to be rHH(AB) = 0.74, rHF(BC) = 1.85, pHH = -0.25, pHF = -0.90.
As the atom F approaches H2, there is fluctuation of potential energy towards the decrease direction while the kinetic energy is also fluctuating but towards the increase direction. The change in the energy can be observed experimentally by measuring the temperature of the reaction. The momentum of HF is still much larger than that of the HH, so the H and F atom vibrate strongly even after the reaction complete and formed as the HF product. That is due to the excess in energy before the reaction is transformed to the vibration energy of the product. The momentum between HH becomes constant after reaction as the H atom move apart from the HF product.
Good. What about using IR? Mak214 (talk) 10:46, 23 May 2019 (BST)
Q9: Discuss how the distribution of energy between different modes (translation and vibration) affect the efficiency of the reaction, and how this is influenced by the position of the transition state.
For the reaction of H2 + F: The graph one left hand side corresponding to higher translation energy and lower vibration energy and the graph on right corresponding to high vibration energy and low translation energy. In this reaction, only the high translation energy one is reactive which indicates a early barrier, early transition state. Translation energy is effective in overcoming the barrier and vibration energy is not.
For the reaction of HF + H:
The graph one left hand side corresponding to higher translation energy and lower vibration energy and the graph on right corresponding to high vibration energy and low translation energy. In this reaction, only the high vibration energy one is reactive which indicates a late barrier, late transition state. vibration energy is effective in overcoming the barrier and translation energy is not.
Good. Mak214 (talk) 10:46, 23 May 2019 (BST)
In conclusion, as Polanyi's empirical rules suggests high translation energy favours reaction with early transition state and high vibration energy favours reactions with the late transition state. And also Hammond’s postulate states that the transition state of a reaction resembles either the reactants or the products, to whichever it is closer in energy. Since H2 + F is an exothermic reaction, early TS is expected and the TS is closer to the reactants in energy. While HF + H is an endothermic reaction, so TS closer to product in energy, late TS state is expected.
Reference
[1] Steinfeld JI, Francisco JS, Hase WL. Chemical kinetics and dynamics. Englewood Cliffs (New Jersey): Prentice Hall; 1989.