MRD:ql2018
Molecular Reaction Dynamics Lab
Exercise 1- (H+H2 System).
Q1ː On a potential energy surface diagram, how is the transition state mathematically defined? How can the transition state be identified, and how can it be distinguished from a local minimum of the potential energy surface?
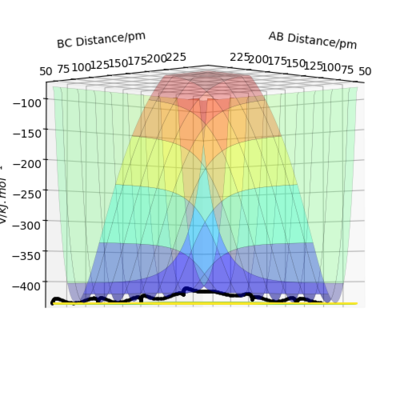
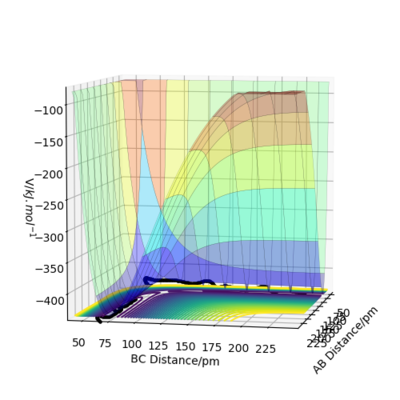
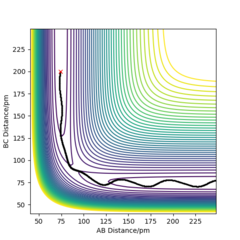
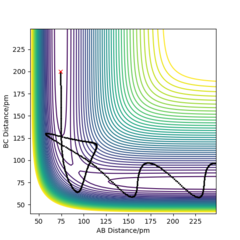
A transition state can be found as a saddle point on the potential energy surface diagram. Mathematically, the transition state is defined as the maximum on the minimum energy path linking reactants and the products. To distinguish the transition state from local minima,this can be done by viewing the potential energy surface from different angles. The transition state is the only point that is a minimum point from one angle(Figure 1)but a maximum from second angle(Figure 2). IOK. Could you use some equations to define this also? Mak214 (talk) 18:23, 29 May 2020 (BST)
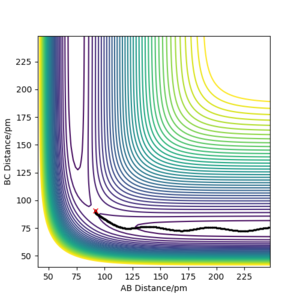
Q1ː Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” plot for a relevant trajectory.
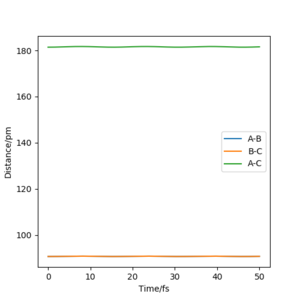
My estimation for the transition state position rts is 90.7pm.Because for the transition state, the potential energies of both p1 and p2 are zero.There is no change of the energy which means that the gradients of potential energy surface is zero and there is no force acting on atoms.So r1 and r2 will keep constant.The graph corresponds should be a straight line since there is no oscillation. Well done. Mak214 (talk) 18:23, 29 May 2020 (BST)
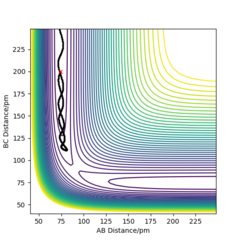
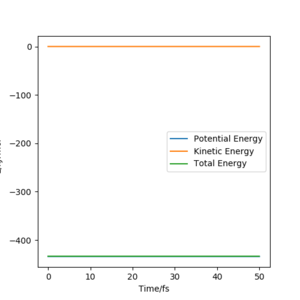
Q3ːComment on how the mep and the trajectory you just calculated differ.
As the two graphs shown below, the difference is obvious that the trajectory on the contour plot of mep is shorter than that of dynamics. And there is also no oscillation compared with the graph generated by dynamics. The reason for this is because that there is zero kinetic energy for mep due to zero velocity and momentum, there will be no gain in vibtational energy as a result. Therefore the trajectory shows no oscillation. Also the change in total energy is different. Since there is no gain in kinetic energy, the total energy will drop as potential energy losses. Yes. in MEP the momenta are reset to zero at every timestep so the system falls into the nearest local minimum. Mak214 (talk) 18:23, 29 May 2020 (BST)
For dynamics, the total energy is conserved as there is gain in kinetic energy when H2 formed.And due to this bond forming, the vibrational energy results in the oscillations on the contour plot.
Q4ːComplete the table above by adding the total energy, whether the trajectory is reactive or unreactive, and provide a plot of the trajectory and a small description for what happens along the trajectory. What can you conclude from the table?
Well done. Mak214 (talk) 18:23, 29 May 2020 (BST)
Q5ːGiven the results you have obtained, how will Transition State Theory predictions for reaction rate values compare with experimental values?
The transition state theory assumes that once the reactants have enough kinetic energy to pass through the transition state. The product must formed and the reaction would not go to reverse. However, the given experimental results indicate that it was not true. The products can recross the transition state and reform reactants. As a result, the transition state theory overestimates the reaction rate compared to experimental results. Also, due to the quantum tunneling effects, atoms with energy lower than activation energy can still pass through the barrier, which leads to a underestimation of reaction rate. Good discussion - you should make reference to the relevant literature here to support what you are saying. Mak214 (talk) 18:51, 29 May 2020 (BST)
Exercise 2ː F - H - H System
Q6ː By inspecting the potential energy surfaces, classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved?
According to the potential energy surface graph F, H2 to H and HFis an exothermic reaction since it has higher potential energy for F and H2. Therefore H + HF to F, H2 is an endothermic reaction. This shows that the H-F bond is stronger than H-H bond. Because the formation of H-F bond releases more energy than the breaking of H-H bond. Good. Could talk about the nature of HH vs HF to bulk out this answer a bit.Mak214 (talk) 19:03, 29 May 2020 (BST)

Q7ː Locate the approximate position of the transition state.
The approximate position of transition state for this reaction is when F-H isr1 = 181.300 pm and H-H isr2 = 74.483 pm. The momentum at this point is zero. Yes. well done. Mak214 (talk) 19:03, 29 May 2020 (BST)
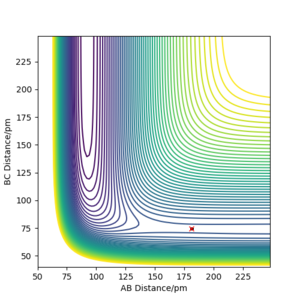
Q8ː Report the activation energy for both reactions.
The potential energy of the reactants F + H2 is -434.625 kJ.mol-1. The potential energy of the transition state is -433.981 kJ.mol-1. The activation energy is equal to the energy of transition state minus the energy of the reactants, which is 0.644 kJ.mol-1.
The potential energy of the products H + HF is -556.231 kJ.mol-1. The potential energy of the transition state is -433.981 kJ.mol-1. The activation energy of this reverse reaction is equal to the energy of transition state minus the energy of the products, which is 122.25 kJ.mol-1.
Well done. Mak214 (talk) 19:03, 29 May 2020 (BST)
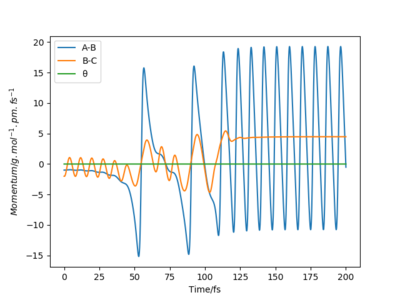
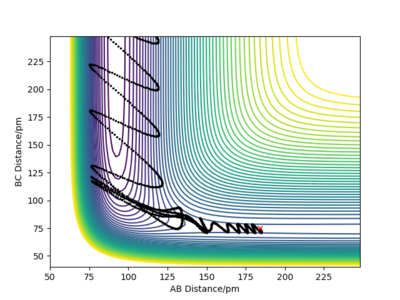
Q9ːIn light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. Explain how this could be confirmed experimentally.
The conditions are set that the the bond distance is 184pm between F and H and the bond distance is 75 between H and H. p1=-1 and p2= -2. From the graph, it can be seen that the product has greater vibration. This is because the potential energy transfer to kinetic energy and transitional energy.
The energy transfer can be confirmed by using IR spectroscopy. Because the vibration of HF bond can be detected by IR. Calorimetry can also be used to detect the heat generated during the reaction. However, this heat includes both vibrational and transitional energy. Yes. Well done. Mak214 (talk) 19:03, 29 May 2020 (BST)
| Momentum vs Time | |
|---|---|
 |
 |
Q10ːDiscuss how the distribution of energy between different modes (translation and vibration) affect the efficiency of the reaction, and how this is influenced by the position of the transition state.
From Polanyi's rule, It is known that the transitional energy can be more efficient for a exothermic reaction as it helps the reactants to pass through early transition state. In endothermic reaction, it has late transition state that the vibrational energy is more effective. It helps the reactants to pass the late transition state.1
Nice report - would have great to have some further discussion, especially in the second part where you talk about polanyi's rules to show that you have thought about how this relates to what you have seen in your own simulations. Mak214 (talk) 19:03, 29 May 2020 (BST)
Reference
1.J.C. Polanyi. Some Concepts in Reaction Dynamics. Science 1987, 236 (4802): 680-690. doi:10.1126/science.236.4802.680