MRD:programmingyay
Molecular Reaction Dynamics
H +H2 system
Dynamics from the transition state region
HA is an atom that collides with the molecule HBHC to form a molecule HBHA and an atom HC in a reversible reaction. Therefore, the initial distance AB will be greater than BC .
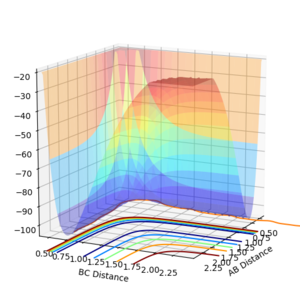
The transition state of this chemical reaction corresponds to the saddle point on the potential energy surface. Plotting the distances AB vs BC vs Potential Energy shows the energy changes as the molecules and atom interact. The transition state can be found by taking the second derivative of the function of the potential energy surface . (Figure 1.) A second derivative value greater than zero corresponds to the saddle point (Transition state), less than zero would be a minima.
Jas213 (talk) 12:17, 11 May 2018 (BST) An Introduction to the topic or the lab would have been nice. You're talking about the second derivative, but what does this imply about the first derivative?
Trajectories from r1 = r2: locating the transition state
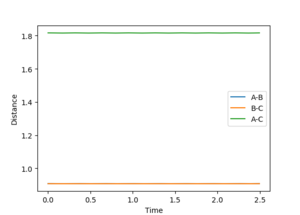
As the system is symetric at the transition state the distances AB and BC must be equal. The AB BC momentum must be zero as there is no force acting on the atoms. Therefore, there is no kinetic energy and all the energy would be potential From this the estimate for the transition state position (rts) was found to be 0.908 Å. A plot of Time vs Inter-nuclear distance shows very little oscillation at this distance. This found by testing points from the blank region on the contour plot (the position of the transition state) and using them to find a straight line on the Time vs Inter-nuclear distance plot. (Figures 2 and 3.)
MEP
The minimum energy pathway (MEP) corresponds to the infinitely slow motion of atoms. This gives a similar graph to (Figure 2.) however, it does not take into account inertial nature of the molecules interacting
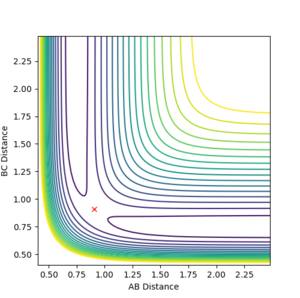
Jas213 (talk) 12:15, 11 May 2018 (BST) Missing a plot here, just saying it's similar to figure 2 doesn't show me what you did.
Reactive and unreactive trajectories
Jas213 (talk) 12:25, 11 May 2018 (BST) Table is overall correct. However, no description of the method. What is now being done? What's the point of the table?
| PAB | PBC | Contour Plot | Energy | Description |
|---|---|---|---|---|
| -1.25 | -2.5 | 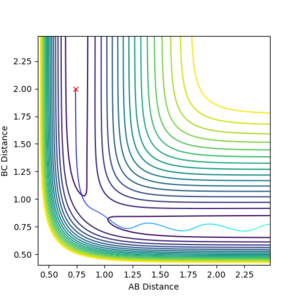 |
Kinetic:+4.687
Potential:-103.706 Total:-99.018 |
Reactive- Reaction path shows that A attack BC when no oscillations (stationary) to form an AB molecule which oscillates. |
| -1.5 | -2.0 | 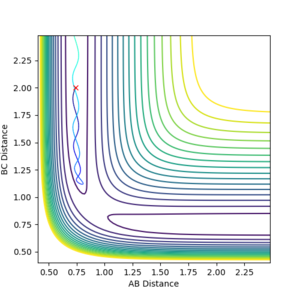 |
Kinetic:+3.250
Potential:-103.706 Total:-100.456 |
Unreactive- Reaction path shows no product formation, as the BC bond length stays the same. A approaches the molecule but does not have sufficient energy to react. Jas213 (talk) 12:25, 11 May 2018 (BST) "Not sufficient energy", What type of energy is not sufficient? Comment on the difference in kinetic energy. |
| -1.5 | -2.5 | 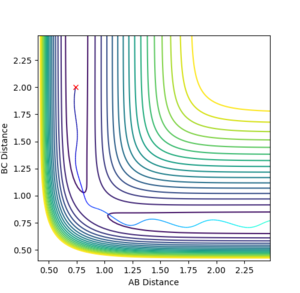 |
Kinetic:+4.750
Potential:-103.706 Total:-98.956 |
Reactive- Reaction path shows product formation as the oscillating A approaches BC to form a new AB molecule which also oscillates (to a lesser extent) Jas213 (talk) 12:25, 11 May 2018 (BST) Why does it oscillate to a lesser extent? |
| -2.5 | -5.0 | 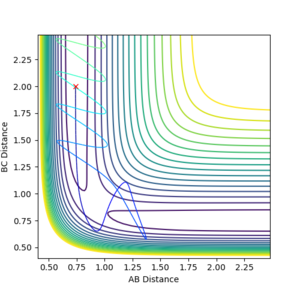 |
Kinetic:+18.750
Potential:-103.706 Total:-84.956 |
Unreactive- Reaction path shows no product formation. A approaches B-C causing vibrations in the molecule. However, it dissociates and doesn't form a new A-B molecule. |
| -2.5 | -5.2 | 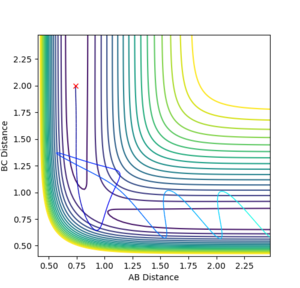 |
Kinetic:+20.290
Potential:-103.706 Total:-83.416 |
Reactive- Reaction path shows product formation. A approaches and a new AB molecule is formed with a much greater degree of vibration. Jas213 (talk) 12:25, 11 May 2018 (BST) No comment on recrossing (transition state being reached twice). Also missing in previous example. |
Jas213 (talk) 12:25, 11 May 2018 (BST) Little comment on what you overall conclude from these examples would have been good/expected.
Transition State Theory
The main assumptions of transition state theory are[1]:
- Reactants and the activated complex (transition state) are in equilibrium but the products and activated complex are not. (Quasi-equilibrium)
- That atomic nuclei behave according to classical mechanics ie. atoms must collide with sufficient energy to form the transition state and react.
- The reaction must pass through the saddle point to proceed.
However, this is not representative of all systems. For instance it does not take into account the effect of tunneling, which would mean the molecules do not have to collide with sufficient energy to overcome the barrier. This is important for reactions with smaller activation energy requirements.
At high temperature higher vibrational modes are occupied resulting in more complex movements and interactions. This means the transition state may not be near the saddle point on a potential energy surface.
When comparing to the data, increasing one of the momenta values and keeping the other steady made the reaction occur if it had not previously reacted.
Jas213 (talk) 12:29, 11 May 2018 (BST) There are some misunderstandings reagrding TST theory here. We would have expected a comment on the statement of TST theory, that if the TS is reached, it will continue to form products. However, in the penultimate example of your previous table, you could see that this is not necessarily true and it might go back to reactants.
F-H-H system
PES inspection
The reaction between H and HF is endothermic. This is due to the strength of the H-F bond, which is caused by the large difference in electronegativity between the H and F atoms. This results in a large positive value for the enthalpy of dissociation, this is a much greater magnitude than the negative value of H-H bond formation. Meaning that energy is required from the surroundings for this reaction to occur. According to Hammond's postulate the transition state of Endothermic reactions resemble the products
Contrastingly, the reaction between HH and F is exothermic. This is also due to the strength of the H-F bond. When formed this has a very large negative enthalpy of formation, which is much greater than the dissociation enthalpy of HH. Overall, energy is given out to the surrounding in this reaction. According to Hammond's postulate the transition state of Exothermic reactions resemble the reactants.
Transition state
For both reactions the same transition state is formed, [F-H-H]‡ . However, the position of the transition state is different for both reactions. For HF+H (Figure 5) and HH + F (Figure 4). Where H-H=1.81Å and H-F=1.74Å, and momenta=0.
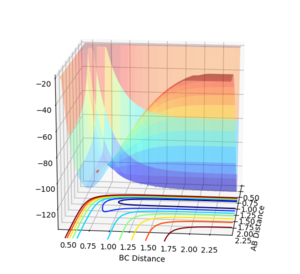
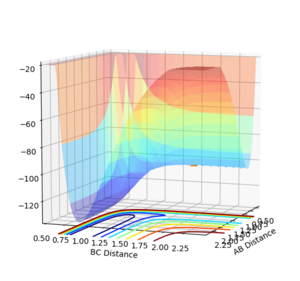
Jas213 (talk) 12:33, 11 May 2018 (BST) How did you find this transition state? What was your method? Where are plots showing that you are sure that this is the correct transition state? Figure 5 shows a slight movement away from your suggested TS, implying that this is not the correct TS. You would have needed to provide a Distance vs. Time plot, which would prove that the distances do not change/are constant.
Activation Energy
Activation energy is the difference in energy between the Transition state and the reactants. For this reaction the Energy of the transition sate was found, using the Energy vs. Time graph of the transition state, to be -103.60 Kcal/mol.
The energy of reactants was found using the bond lengths of the reactants for both molecules. For the FH + H reaction this was -134.21 Kcal/mol and for the HH + F reaction this was -104.44Kcal/mol
Using this the activation energies could be calulated:
- For FH + H reaction -103.60 Kcal/mol - (-134.21 Kcal/mol)= +30.61Kcal/mol
- For HH + F reaction -103.60 Kcal/mol - ( -104.44Kcal/mol)= +0.84Kcal/mol
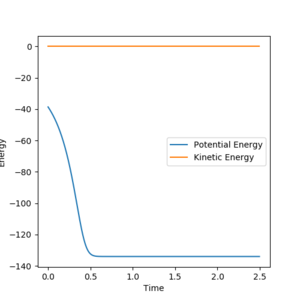
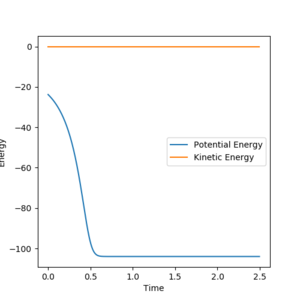
Reaction Dynamics
For the reaction F+HH the relative trajectory was analysed from the starting point of the initial inter-nuclear distances of r(F-H)=2.3Å and r(H-H)=1.8Å, the momentum values were then changed until a reaction could be observed. In this case the momenta values were: p(F-H)=-5 and p(H-H)=0.1
As this is an exothermic reaction energy would be expected to be released into the surroundings. This is likely expressed by the oscillation of the F-H molecule formed as the kinetic energy increases, coupled to a decrease in potential energy (conservation of energy).
The reaction could be monitored using Calorimetery by measuring the enthalpy change.
Polanyi's rules state that kinetic energy more effective than translational energy at activating a late transition barrier, and conversely translational energy is more effective for early transition barriers. [2]
This means that when kinetic energy is increased relative to translational energy for an exothermic reaction, the reaction is more likely to take place due to the late transition state. So for the FH +H system translational energy is favoured and for the F +HH system kinetic energy is favoured.
Jas213 (talk) 12:38, 11 May 2018 (BST) *calorimetry. Overall, read over your report before submitting it. Some full stops were missing and endothermic and exothermic are adjectives and don't require capitals. Overall a solid and honest attempt at completing the report. All questions attempted, showing engagement, even if with some major misunderstandings/errors.
References
- ↑ Pechukas, P. (1981) ‘Transition State Theory’, Annu. Rev. Phys. Chem., 32(1), pp. 159–177. doi: 10.1146/annurev.pc.32.100181.001111.
- ↑ Zhang, W., Kawamata, H. and Liu, K. (2009) ‘CH stretching excitation in the early barrier F + CHD3reaction inhibits CH bond cleavage’, Science. American Association for the Advancement of Science, 325(5938), pp. 303–306. doi: 10.1126/science.1175018.
