MRD:pp4717
On a potential energy surface diagram, how is the transition state mathematically defined? How can the transition state be identified, and how can it be distinguished from a local minimum of the potential energy surface?
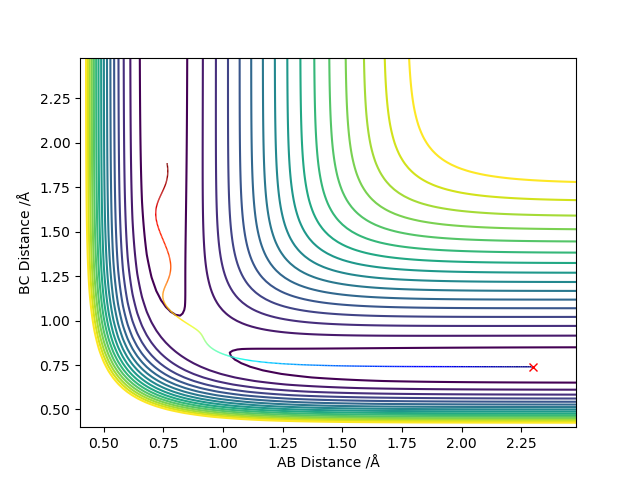
The transition state is generated from making a skew plot to form coordinates q1 and q2. The coordinates q1 and q2 are then differentiated and the minimum of one curve is the maximum of the other. The point at which the minimum and maximum converge is the transition state.
(Some more details of this definition are required here.) Cq3417 (talk) 01:03, 30 May 2019 (BST)
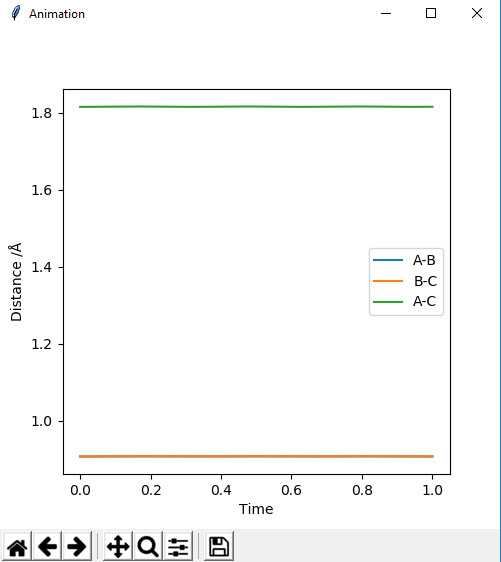
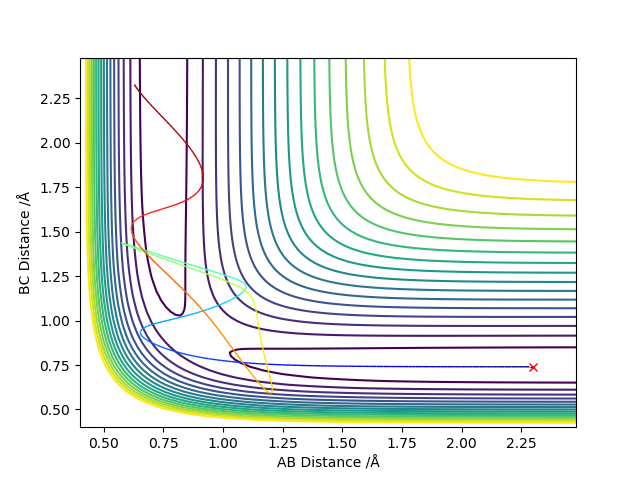
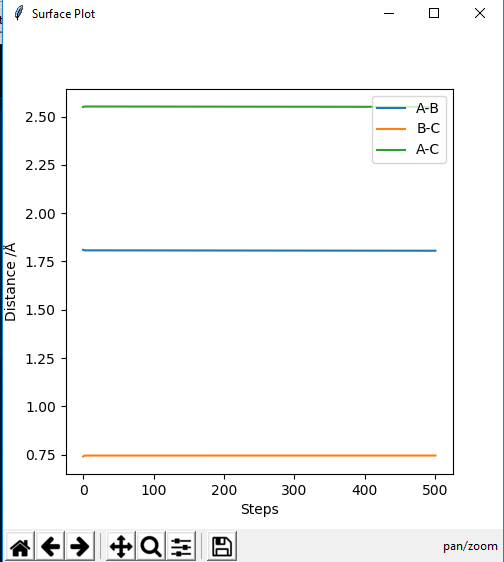
Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” plot for a relevant trajectory.
The best transition state position was 0.9075 Ǎ
The Internuclear Distances vs Time plot shows two straight lines corresponding to the bonds A-C and B-C which helped to identify the transition state. There are no fluctuations of the distance with time of the bonds in the transition state because they have no potential energy.
(Any comment with the aid of contour plot?) Cq3417 (talk) 01:03, 30 May 2019 (BST)
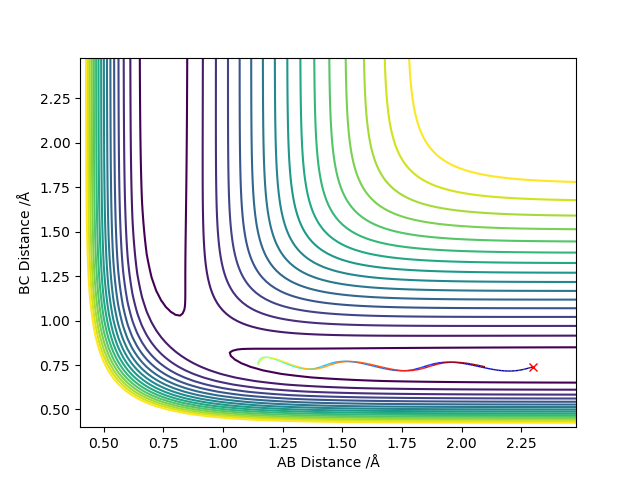
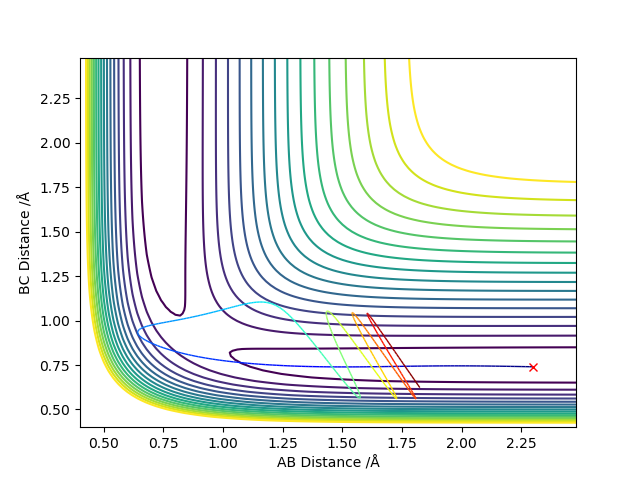
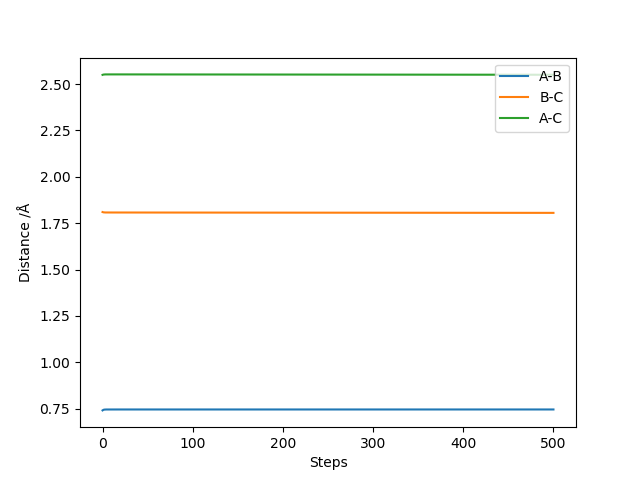
Comment on how the mep and the trajectory you just calculated differ.
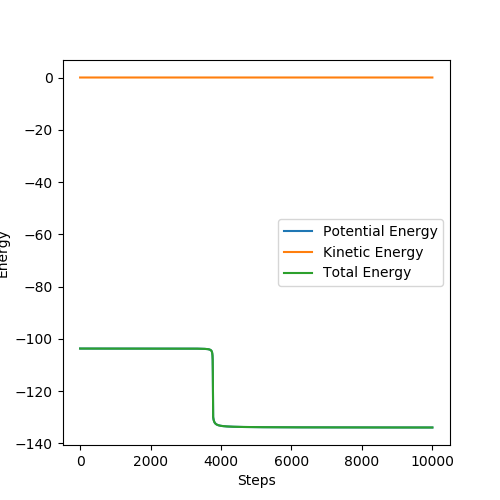
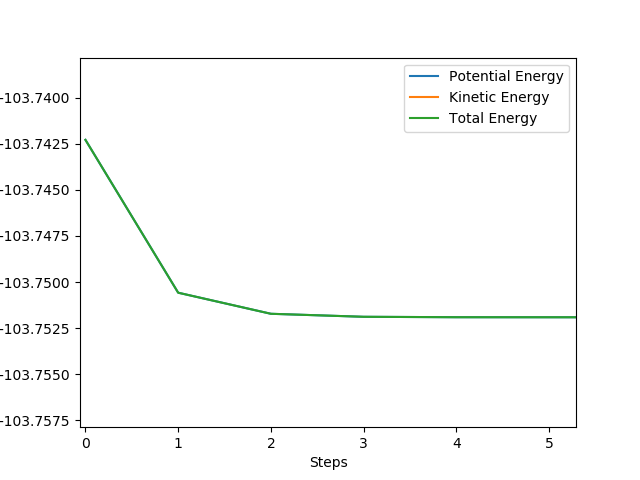
MEP does not consider the momentum that the molecule already has, only the momentum that was defined in the initial conditions in the first step. In the dynamic trajectory, the residual momentum is considered which is why there are vibrations present and the point at which the potential energy is zero occurs at a larger distance.
(Again, more details are required here.) Cq3417 (talk) 01:03, 30 May 2019 (BST)
(You missed a question here.) Cq3417 (talk) 01:03, 30 May 2019 (BST)
By inspecting the potential energy surfaces, classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved?
F + H2 is an exothermic reaction; this shows that the bond strength is strong.
H + HF is endothermic; this shows that the bond strength is weak.
Locate the approximate position of the transition state.
For the F + H2 reaction:
The transition state is located at 1.83 Ǎ.
For the H + HF reaction:
The transition state is located at 1.802 Â
(My question is how did you get these results?) Cq3417 (talk) 01:03, 30 May 2019 (BST)
Report the activation energy for both reactions.
For the F + H2 reaction:
The activation energy for the F + H2 reaction = 29.474 KCal/mol
For the H + HF reaction:
Activation energy = 0.009 KCal/mol
(Any more comments instead of just presenting the values?) Cq3417 (talk) 01:03, 30 May 2019 (BST)
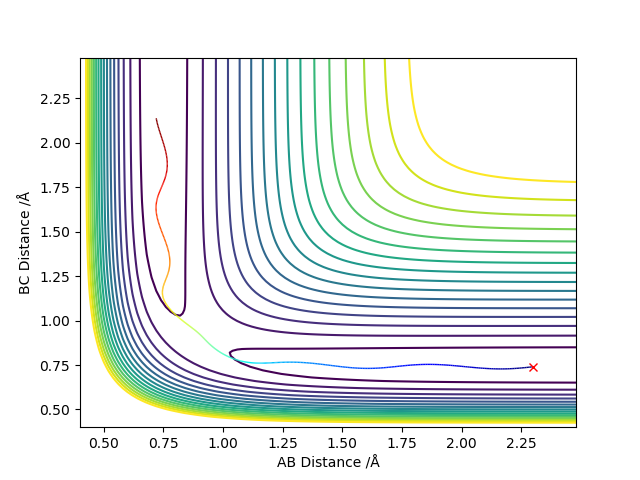
In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. Explain how this could be confirmed experimentally.
These set of initial conditions give the reactive trajectory for the F + H2 reaction.
(This answer is completely insufficient for this question.) Cq3417 (talk) 01:03, 30 May 2019 (BST)
(A question is missing here.) Cq3417 (talk) 01:03, 30 May 2019 (BST)
(Overall, this is an uncompleted report with two missed questions and insufficient discussion.) Cq3417 (talk) 01:03, 30 May 2019 (BST)