MRD:oirewajgoijreoij234502850437603284
This is a good report. You answered majority of this report fully. I would say there a few sections in the middle that could have used further detail. But beyond that it was great. Well done :) Mys18 (talk) 23:06, 1 June 2020 (BST)
Transition State
Explanation
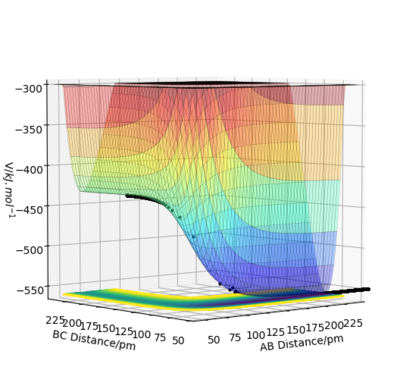
On a potential energy surface, a transition state is a stationary point:
,
and further:
It can be identified as a maximum in along the bond direction and minimum in the direction orthogonal to the bond. The transition state is different from a local energy minimum, the local energy minimum is given by the condition:
and

A local minimum on the potential energy surface can be distinguished because all the points around it will have larger values but for a saddle point some point will have larger values and others have smaller values.
IF you mean positive for 'larger' and negative for 'smaller' then, good. What is the saddle point? Mys18 (talk) 22:46, 1 June 2020 (BST)
Transition state position
The estimate for the transition state position is where . This is a position where there is no force acting along AB or BC, the atoms A, B, C are stationary, there is no kinetic energy and only potential energy.

Reaction path trajectories
In minimum energy path (mep) calculations, the hydrogen atoms in the molecule formed does not vibration as they move away, the reaction path is a straight line. In Dynamics calculations, the molecule vibrates as it move away, the reaction path is wiggling.
Good observation. Why is it that MEP is more linear whereas dynamic is wavy? Does one consider the vibrational energy, how does the other differ Mys18 (talk) 22:50, 1 June 2020 (BST)


Reactive and unreactive paths
From the conditions shown above, we can see that whether a reaction path is reactive or not does not necessarily depends on whether the molecules have enough energy to cross the transition state but importantly, it depends on the kinetic energy distribution between the products, their velocities and momentum. Even if the total energy of a system is large enough, the reaction may still not be reactive.
Transition State Theory
The Transition State Theory will overestimate the rate of reaction compared to experimental values because an important assumption in the Transition State Theory is all the trajectories along the reaction coordinate with kinetic energy greater than the activation energy will be reactive. But we have seen in the above examples that this is not the case and they are not all reactive.
Precisely! It is important to consider other assumptions of TST, what about non-classical tunnelling, is this in line with TST? Mys18 (talk) 22:54, 1 June 2020 (BST)
H-F-H system
The H-F-H system is a symmetric molecule, the reactant and the product are the same, therefore, the transition state is also symmetric. There will be no force acting along AB or BC. The distance between H-F is found to be:
F-H-H system
The F + H2 reaction is exothermic as can be seen from the potential energy surface. The H-H bond is broken and a new H-F bond is formed during the reaction. This sugguests the H-F bond is stronger than the H-H bond. According to Hammond's postulate, the structure of the transition state will resemble the structure of reactant or product that is closer in energy. So, the transition state of the reaction will be similar to the reactant when H2-H3 distance is small and F1-H2 distance is large. The transition state is early and it will be relocated closer to the reactant.

The transition state of the reaction is found to be approximately:
Nice! Mys18 (talk) 22:57, 1 June 2020 (BST)
Activation energy
The activation energy is the energy difference between the transition state of a reaction and the energy of the reactant which is not kinetic.
The activation energy of the reaction is found by setting the system near the transition state at and let the system to go back to the reactants and then find the total energy of the system. Kinetic energy = 0, all the energy are potential energy.
Activation energy =

H-H-F system
This system is the reverse of the F-H-H system that a H atom collide with the H-F molecule, the H-F bond is broken and a new H-H bond is formed. From the potential energy surface plot of this reaction we can see that this reaction is endothermic and this shows H-H bond is weaker than the H-F bond. According to the Hammond's postulate, the transition state will resemble the structure of the product and so the distance between H1-H2 is small and the distance between H2 and F3 is large.

The transition state of the system is found to be approximately:
The activation energy of the reaction is found when the reactant is slightly displaced from the transition state and let it go back to the starting position and calculated using MEP.
The activation energy is calculated as:

REally good job on this section. methodology, values and units spot on. Mys18 (talk) 22:58, 1 June 2020 (BST)
Release of the reaction energy
The total energy of the reaction is conserved, when the F atom and a H2 molecule collided with each other, the H2 atom will bounce back and forth between the F1 atom and the H3 atom, whether the reaction trajectory is reactive or not depends on how much the initial kinetic energy of the F atom and H2 molecule have and how far the H3 atom is bumping away from F1 and H2. Initially, the system composed of F1 atom and H2 molecule, there will be no change of dipole moment when the H2 molecule vibrates and is IR inactive. If the reaction path is reactive, the product will be a H atom and a H-F molecule. If the H-F molecule vibrates, there will be a change in dipole moment and therefore IR active. We could observe a band due to the transition from v0 to v1 vibrational state and 1st and 2nd overtunes of the vibration. So the release of reaction energy can be measured by IR spectroscopy. Also, when a molecule vibrates, it radiates energy in the range of IR and the radiation can be detected. Also, calorimetry can be used to monitor the reaction as the reaction is exothermic and temperature will increase. If the reaction trajectory is not reactive, the reactant will remain the same so it will not be IR reactive.
Interesting. What could you use to measure the emission of infrared radiation - chemilumi_ _ _ _ _ _ _ _. Mys18 (talk) 23:00, 1 June 2020 (BST)
Reactive trajectory

Unreactive trajectory

Distribution of energy, position of transition state
The distribution of energy such as translational and vibrational energies between the atoms and molecules in a system is important to whether a reaction trajectory is reactive or not. If in the system there is little kinetic or vibrational energy, the atom cannot cross the transition state because it does not have enough energy. Once the system has enough energy, the distribution between translational, vibrational and potential energy is important. After a system have enough energy to overcome the activation energy barrier, the excess energy the system possess will allow the middle atom to bounce back and force between the first and the third atom. Whether the second atom will stay with the first or the third atom depends on how much excess energy it has and therefore determines whether a new bond will form or not. As the excess energy increases incrementally, whether the reaction trajectory is reactive or not alternates. As shown in the exercise, an F atom and a H2 molecule approaching each other with little translational energy but enough energy above the activation energy barrier can lead to the formation of the product. There is almost no vibrational energy, once the H2 atom is pulled towards the F1 atom, it stick with it and does not bounce back.

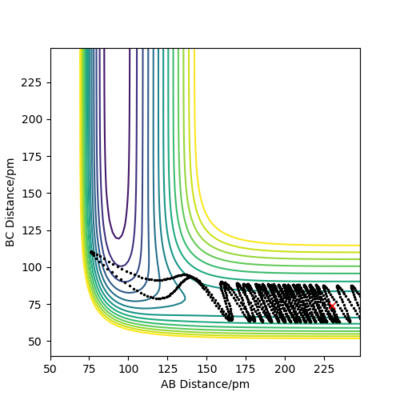
Good. Mys18 (talk) 23:05, 1 June 2020 (BST)
Polanyi's empirical rules and Hammond's Postulate
When the transition state of a reaction is not symmetric a in the case of F + H2 and H + H-F systems, the transition state will change its position. In the F + H2 system, the transition state is a early transition state, it is closer to the reactant than the product. Translational energy will make the reaction more efficient because it helps to go across the transition state In the H + H-F system, the transition state is a late transition state, the transition state is close to the product than the reactant. Vibrational energy in the reactant will increase the efficiency of the reaction as it helps to go beyond the transition state while a translational energy will bounce back. This rule is closely related to the Hammond's postulate in determining whether the transition state will be like the reactant or the product according to whether the reaction is exothermic or endothermic.1
GReat work explaining the rule with context. Mys18 (talk) 23:05, 1 June 2020 (BST)
References
1. J. C. POLANYI, Science (80-. )., 1997, 236, 680–690.





