MRD:nk1016
Molecular Reaction Dynamics
Exercise 1: H + H2 System
Question 1: The gradient is 0. The minima and the transition structures can be distinguished by using the 2nd derivative of the potential energy as the value at gradient = 0 will be positive (upwards curvature) and negative (downwards curvature) respectively.
Jas213 (talk) 17:13, 25 May 2018 (BST) very short, no equations, not specifically mentioning that we are dealing with a three dimensional problem.
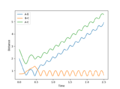
Question 2: My best estimate for the transition state position is 0.9159888. Below is the Internuclear Distance vs Time graph for r1 =r2 = 0.9159888 and p1 = p2 = 0. This shows no movement which should occur at the transition state as neither the products nor the reactants are formed.
Jas213 (talk) 17:13, 25 May 2018 (BST) what's the unit of this ? what position do you mean? You should be more precise and talk about what you mean by r1 and r2. What reaction are you looking at? How did you find this distance?
Question 3: With the mep, the atoms stay relatively close together but with dynamic, once the incoming hydrogen atom has come in, the displaced hydrogen atom shoots off.
Question 4: Changing the initial conditions makes it so that the other atom is displaced. Changing the sign makes it so particle A approaches BC and there is some interaction but bounces off.
Question 5: With r1 = 0.74 and r2 = 2.0, trajectories were run with the following momenta:
| p1 | p2 | Energy (Total) | R/UR | |
|---|---|---|---|---|
| A | -1.25 | -2.5 | -99.018 | R |
| B | -1.5 | -2.0 | -100.456 | UR |
| C | -1.5 | -2.5 | -98.956 | R |
| D | -2.5 | -5.0 | -84.956 | R/UR |
| E | -2.5 | -5.2 | -83.416 | R |
A) Hydrogen A approaches BC and displaces C.
B) Hydrogen A approaches BC but bounces off without affecting BC.
C) Hydrogen A approaches BC and displaces C.
D) Hydrogen A approaches BC and displaces C for a small period of time but C comes back and displaces A.
E) Hydrogen A approaches BC and displaces C but C immediately displaces A before being displaced once more by A so AB is left over.
Jas213 (talk) 17:18, 25 May 2018 (BST) Very basic description. You should have talked about the different proportions of p1 and p2, and how these affect the proportions of translational and vibrational energy. An overall concluding comment on what you learned from this table would have been expected. The picture would have been much clearer if you had given a contour/surface plot in addition to the distance vs. time plots? It looks a bit like you didn't understand these plots.
Question 6: Transition state theory assumptions:
-The intermediates of the reaction are not short-lived.
-Particles obey classical mechanics: This leads to the reaction rate values being smaller than experimental values as effects such as quantum tunneling of electrons are ignored.
-Low Temperature: The theory assumes that the reaction goes along the lowest energy pathway but at higher temperatures, the reaction could possibly go through different pathways.
Jas213 (talk) 17:18, 25 May 2018 (BST) What's your reference for this? You should have commented how the recrossing observed in the previous table disagrees with bullet point number two.
Exercise 2: F-H-H system
Question 1: F + H2 is an exothermic reaction (products more stable than reactants) while H + HF is an endothermic reaction (vice versa). This relates to the H-F bond being stronger than the H-H bond so more energy is released/required when the bond is formed/broken (respectively).
Jas213 (talk) 17:29, 25 May 2018 (BST) Endothermic/Exothermic is correct. Why is the HF bond stronger? You could have either explained it or stated the bond energies.
Question 2: Using Hammond's Postulate, as the transition state would resemble F and H-H more than F-H and H as it would be closer in energy to the former, r1 was set to 0.74 (approximate H-H bond length) and r2 was varied until the atoms stayed in the same relative position. This was at r2 = 1.8135. Below is the Internuclear Distance vs Time graph for r1 = 0.74, r2 = 1.8135 and p1 = p2 = 0.
Jas213 (talk) 17:30, 25 May 2018 (BST) Good that you said something about your method. However, the distance vs. time plot still shows some vibrations, so you are still slightly away from the TS.
Question 3: The (potential) energy at the transition state is -103.743. The activation energy is the energy difference between the reactants and the transition state. For the forward reaction, as the energy of the reactants is -104.02 giving an Ea of 0.277. For the reverse reaction, as the energy of the reactants is -133.955 giving an Ea of 30.212. The energies were identified by setting the fluorine atom away from the hydrogen molecule and then the hydrogen atom from the HF molecule (r2 = 20, r1 = 0.74 and r2 = 0.91, r1 = 20).
Jas213 (talk) 17:29, 25 May 2018 (BST) What are the units? This leads to the correct results and was a successful approach, however we asked you to use the MEP and provide plots of the MEPs as well.
Question 4: The release of reaction energy will be as thermal energy which can be confirmed experimentally using a calorimeter.
Question 5:
Forwards Reaction:
Using r1 = 0.74 (H-H), r2 = 2 and p2 = -0.5 (F-H) , the p1 (H-H vibrational energy) was varied to see how this affected the reaction.
A) p1 = -3: The fluorine atom bounces off - no reaction.
B) p1 = -2.5: The fluorine atom bounces off - no reaction. C) p1 = -2: The fluorine atom displaces the hydrogen - reaction occurs.
D) p1 = -1.5: When the fluorine atom approaches H2, it almost bounces off but succeeds in displacing the hydrogen - reaction occurs.
E) p1 = -1: When the fluorine atom approaches H2, there is some interaction but the fluorine ends up bouncing off - no reaction.
F) p1 = -0.5: All 3 atoms are vibrating together before the fluorine atom ends up displacing the hydrogen - reaction occurs.
G) p1 = 0: This is similar to p1 = -0.5, the reaction occurs. H) The positive values of the momenta give similar results to the negative with the exception of p1 = 1 where the fluorine atom bounces of the H2 but eventually displaces the hydrogen atom.
Changing p2 to -0.8 (increasing translational energy) and p1 to 0.1, a reaction occurs with the fluorine displacing the hydrogen.
These show that having a greater distribution of energy with the translation of the F atom compared with the vibrational energy of the H2 improves the efficiency of the forward reaction with the fluorine displacing the hydrogen quicker.
Reverse Reaction:
Using r1 = 0.91 (H-F) and r2 = 2, p1 and p2 were varied to see what it would take for the reaction to occur.
With p1 = -0.1, p2 = -15: The hydrogen atom comes in but the 3 atoms become separate from each other. When the momentum of the incoming hydrogen atom (p2) is changed to -10, the reaction occurs with the fluorine being displaced. The reaction can also occur if the momentum of the HF bond (p1) is changed to -10 instead. This shows that a greater distribution of energy with the vibration of the HF molecule compared with the translational energy of the hydrogen atom improves the efficiency of the reverse reaction.
Polanyi's empirical rules state that the vibrational energy is more important than translational energy for promoting an efficient reaction with a late transition state and vice versa. This is supported here as fluorine displacing hydrogen has an early transition state and the translational energy had a better effect on the efficiency of the reaction than the virational and the opposite occurs with the hydrogen displacing the fluorine which has a late transition state.
Jas213 (talk) 17:29, 25 May 2018 (BST) You didn't provide a single reference.