MRD:mz5717
H + H2 SYSTEM
Transition State Determination
On a potential energy surface, the transition state is represented by a saddle point, which is mathematically defined as:
When the partial derivatives Vr1 and Vr2 both equal to 0 at a point, if the value of (Vr1r1Vr2r2 - V2r1r2) < 0 at that same point, then it is a saddle point.
Alternatively we can identify it by computing the second partial derivatives, Vr1r1 and Vr2r2. For a saddle point, one of them will be positive and the other one negative.
Additionally, when (Vr1r1Vr2r2 - V2r1r2) > 0, the point represents a local maximum/minimum. It is a local maximum if Vr1r1 < 0, or a local minimum if Vr1r1 > 0.
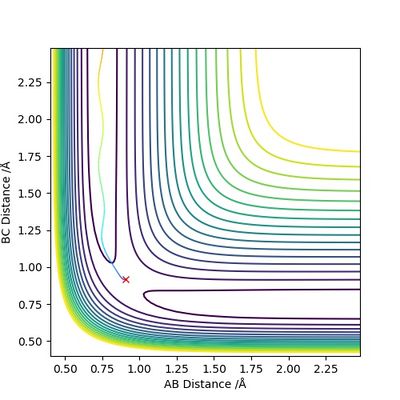
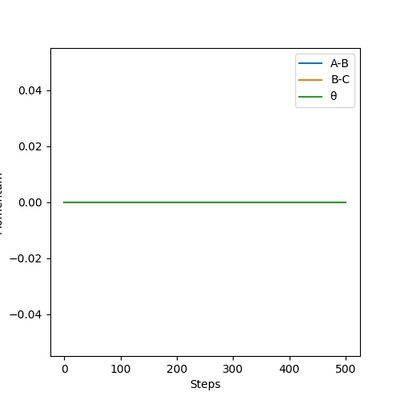
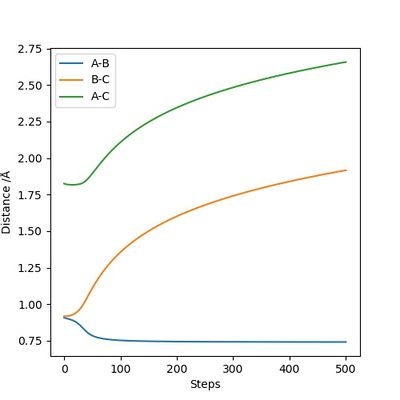
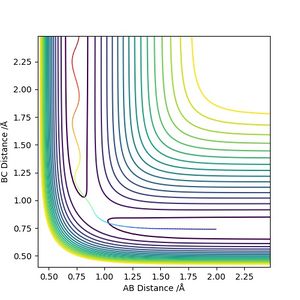
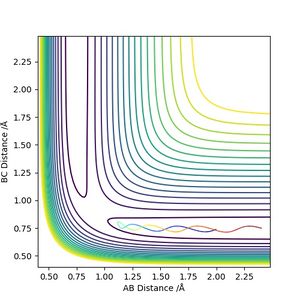
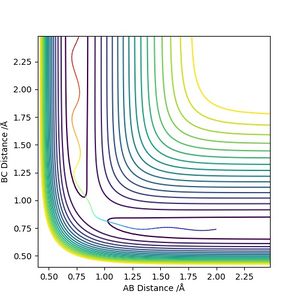
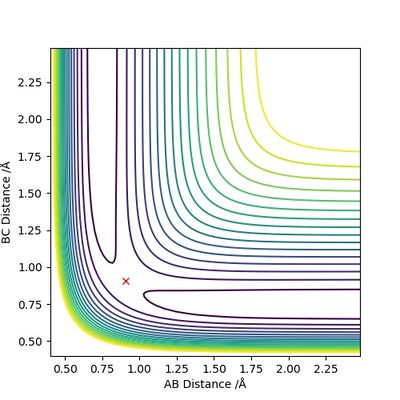
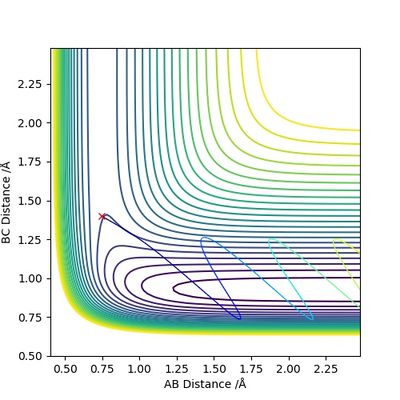
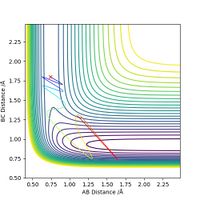
The transition state position is estimated to be 0.90743 Å. i.e. r1 = r2 = 0.90743 Å, p1 = p2 = 0. When subjected to this initial condition, the internuclear distances do not vary with time because the equilibrium established at the saddle point does not provide driving force for any forward or backward reaction.
This is illustrated with the plot below. The contour map shows the position of the TS with a cross.
(Reasonable description of the transition state and good illustration of plots) Cq3417 (talk) 15:37, 15 May 2019 (BST)
 |
 |
Reaction Trajectories
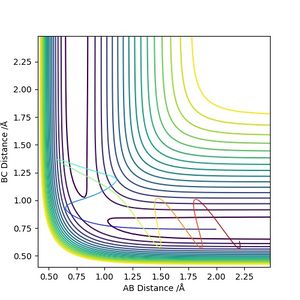
Dynamics and MEP are two methods of calculating the reaction trajectory. The table below compares the outcomes produced by these two methods.
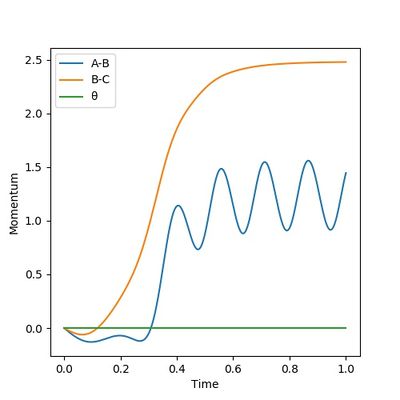
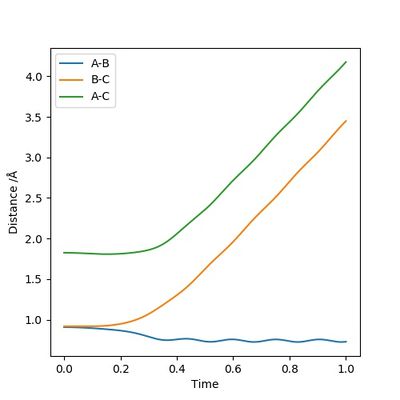
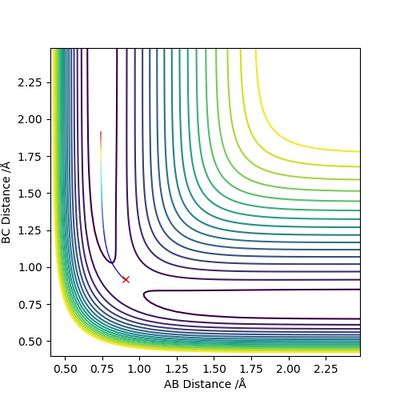
According to Figures 4 and 5, we set the initial conditions to be r1 = 0.74895, r2 = 1.0 (an arbitrary large distance), p1 = -1.2745, p2 = -2.4889, which corresponds with the final position of the trajectory only with the signs of the final momenta reversed. The resulting trajectory is almost the same as those we obtained previously.
(Good explanation.) Cq3417 (talk) 15:37, 15 May 2019 (BST)
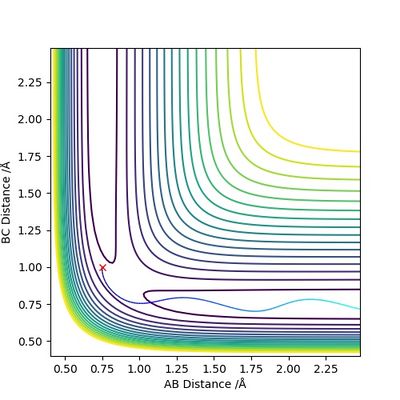
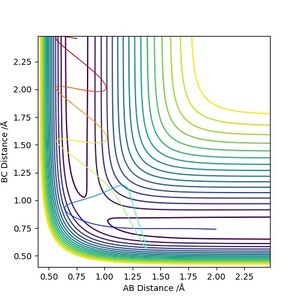
Next, we examine the effect of initial momenta and total energy on the reaction trajectory, i.e. if it is reactive or not, by giving the reactants varying amounts of initial momenta. The results are summarized by the table below.
(Excellent illustration though additionally showing the energy vs time plot can make it better.) Cq3417 (talk) 15:37, 15 May 2019 (BST)
Transition State Theory
The main assumptions of Transition State Theory are:
- The nuclei behave classically
- The reaction path passes through the saddle point representing the transition state
- Once the reaction passes through the TS, it will proceed to the formation of the product(s)
Under most circumstances, TST matches with experimental results to a good extent. However, from the simulation we can conclude that when the kinetic energy supplied is way above the activation energy, this is not necessarily the case. Here the experimental position of the transition state may largely deviate from the theoretical one represented by the saddle point on the potential energy surface. Because of the abundance of energy supplied, more molecules are able to occupy vibrational modes of higher energy, making their motion more complicated and harder to predict. This could be the underlying reason for the large deviation. It could be hard as well to predict the reaction rate under such circumstances; for example, when there is a
reverse reaction going on.
(I think you may need to cite the description of this assumption.) Cq3417 (talk) 15:37, 15 May 2019 (BST)
F - H - H SYSTEM
Transition State Determination
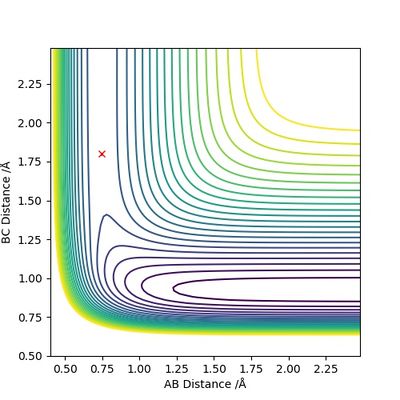
F + H2 --> H + HF is exothermic and has an early TS. The reaction releases energy because the new H-F bond formed is stronger than the H-H bond broken (F is much more electronegative than H). On the other hand, H + HF --> F + H2 is endothermic and has a late TS. The reaction requires input of energy because the H-F bond broken is much stronger than the H-H bond formed.
Setting initial momenta to be both zero, the position of the TS is estimated to be r1 = 0.745 Å and r2 = 1.80 Å.
(Good, nice and clear.) Cq3417 (talk) 15:37, 15 May 2019 (BST)
 |
 |
Activation Energy
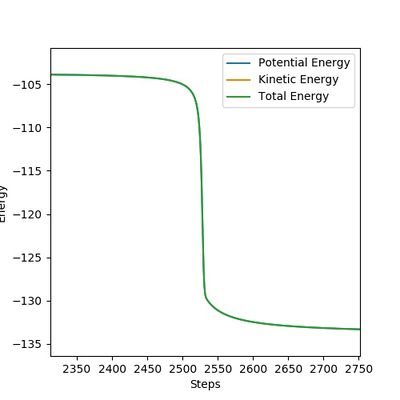 |
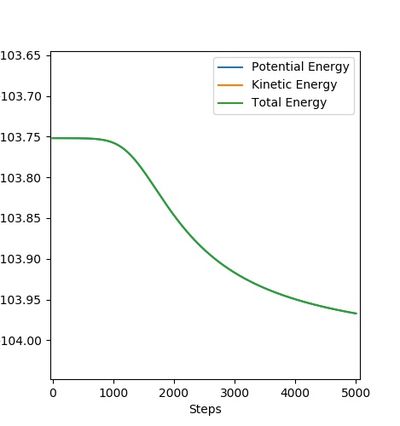 |
The figures above show the energy profiles of H + HF and F + H2 respectively.
The activation energy of H + HF is determined as -103.867 - (-133.257) = 29.390 kcal/mol
The activation energy of F + H2 is determined as -103.752 - (-103.967) = 0.215 kcal/mol
(How you get this plot is my question so I think you need to discuss this in detail.) Cq3417 (talk) 15:37, 15 May 2019 (BST)
Energy Distribution
First we focus on energy conversion. Below is a general case of a successful reaction.
 |
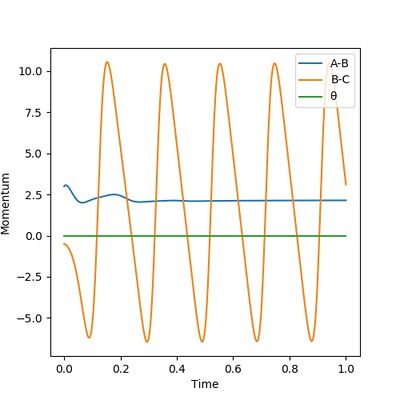 |
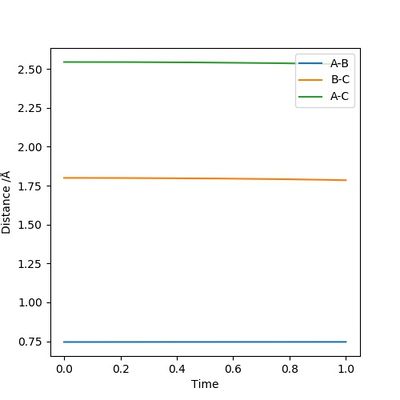
Figure 15 shows that the vibration of bond A-B gradually ceases, which means that bond A-B corresponds to the H-H bond broken. In this case bond B-C must be the new H-F bond formed. The H-F bond continues its vibration and eventually becomes the only source of momentum, which means that the excess kinetic energy from the F atom was converted to vibrational energy after the reaction has occurred. The extent and modes of the H-F bond vibration can be measured by Raman spectroscopy.
(It can be more clear if you include the energy vs time plot here.) Cq3417 (talk) 15:37, 15 May 2019 (BST)
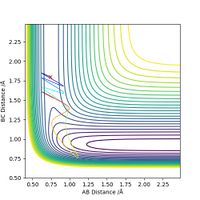
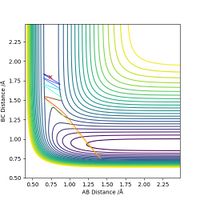
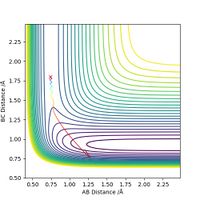
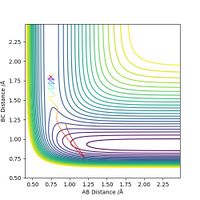
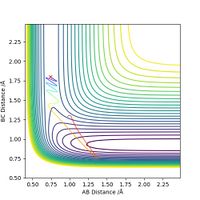
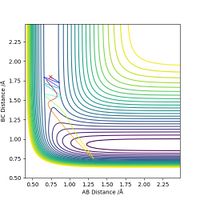
Next, for the reaction F + H2 --> H + HF, we set the initial conditions to rHH = 0.74, rHF = 1.80 and pFH = -0.5. Different values of pHH, ranging from -3 to 3, are tested and the results are summarized in the table below.
 |
 |
 |
 |
 | |
 |
 |
 |
 |
 |
 |
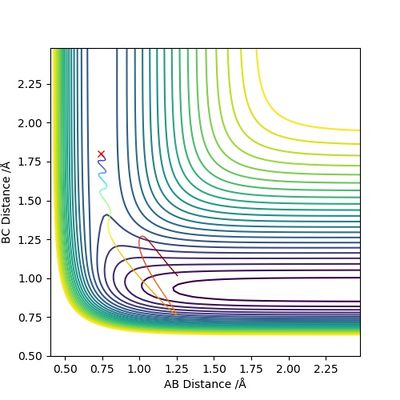
The table shows that when pHH has a value between -1.2 and 0, the reaction proceeds to the product side. In all other cases the reaction trajectory more or less bounces back to the reactant side, and the extent of this backward conversion increases as the absolute values of pHH increases. The only outlier is pHH = 2.4, where the trajectory generally stays on the product side.
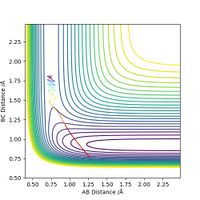
Now increase pHF slightly to -0.8, and largely reduce pHH to 0.1. The trajectory below shows that despite having considerably lower total energy, the reaction still proceeds to the product side.
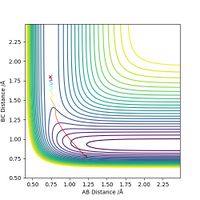
Finally, for the reaction H + HF --> F + H2, we assume that there is very little vibration within the H-F bond (i.e. pHF = 0.2), and that the H atom is approaching with extremely high kinetic energy (pHH = 5). With the initial conditions rHH = 0.745, rHF = 1.80, the trajectory below is obtained. It is evident that the high kinetic energy possessed by the H atom is initially able to break the H-F bond and push the system to the product side; however, HF is reformed afterwards and then the entire process repeats. This is an oscillating reaction.
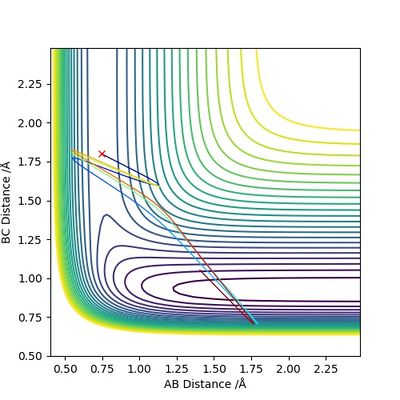
By decreasing pHH and increasing pHF, it is possible to obtain a reactive trajectory. For example, when we let other variables stay constant and change the rest to pHF = 0.3, pHH = 2.6, the reaction can indeed proceed to the product side as shown in the figure below.

(Where is your discussion that respects to the Polanyi's empirical rules?) Cq3417 (talk) 15:37, 15 May 2019 (BST)
In conclusion, a proper distribution of kinetic and vibrational energy is crucial for the trajectory to be reactive. Generally in most cases, a high kinetic energy nearly guarantees a reactive path. The reaction F + H2 --> H + HF has an early TS, and does not need much energy to reach it; the other reaction H + HF --> F + H2 has a late TS, and requires much higher vibrational energy to reach it. The second reaction also shows us that just having high kinetic energy is not enough to trigger a successful reaction. By lowering the kinetic energy and increasing the vibrational energy we see that there is a delicate balance between the two that eventually leads to a reactive trajectory.
(Overall this is a sufficient report that shows a good understanding of this concept. However, the citation of reference and, the combination of theories and experimental data are required to be noticed.) Cq3417 (talk) 15:37, 15 May 2019 (BST)