MRD:msk315
Exercise 1: H + H2 system
What value does the total gradient of the potential energy surface have at a minimum and at a transition structure? Briefly explain how minima and transition structures can be distinguished using the curvature of the potential energy surface.
At both minima and the transition structure, the gradient is 0. However, they can be distinguished by the values of the second derivatives.
The second derivative of minima will be greater than zero. In the potential energy surface, it will appear as a hole.
For the transition structure, the second derivative will be less than zero. In the potential energy surface, it will appear as a saddle point.
(Fv611 (talk) 15:41, 17 May 2017 (BST) Good. "Hole" is not the correct term, try "well" next time.)
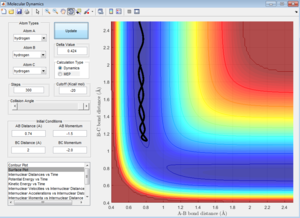
Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” screenshot for a relevant trajectory.
In order to estimate the transition state, the graph of "internuclear distances vs time" was investigated. When the 3 hydrogen atoms are placed at a particular distance where r1=r2 with no initial momentum, then there should be no particular movement or oscillation. The transition state position is estimated to be rts=0.908 A. At this point, there was the least oscillation.

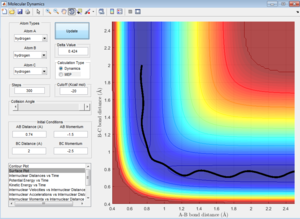
Comment on how the mep and the trajectory you just calculated differ
In mep, the velocity is set to 0. The atom accelerates and moves in the steepest slope. It follows a curved path that corresponds to the minima of the potential energy surface. In Dynamic trajectory, the atom does have an initial velocity and the initial velocity combines with the the acceleration arising from the steepest slope. This results in the oscillating path. To sum up, dynamic gives an oscillating trajectory. However, the mep gives a straight line trajectory, following the floor of the valley.
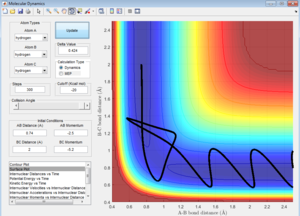
Complete the table by adding a column reporting if the trajectory is reactive or unreactive. For each set of initial conditions, provide a screenshot of the trajectory and a small description for what happens along the trajectory.
(Fv611 (talk) 15:41, 17 May 2017 (BST) Correct, but you do not consider the energy of the reactants. Why are cases 2 and 4 different?)
State what are the main assumptions of Transition State Theory. Given the results you have obtained, how will Transition State Theory predictions for reaction rate values compare with experimental values?
Assumes that the atoms obey classical mechanics, and hence ignores quantum effect such as quantum tunnelling. Hence, it is inaccurate for light systems such as hydrogen, since quantum mechanical influences become important. Assumes that each step in the reaction is long lived enough to reach a Boltzmann distribution of energies. some steps will have different energies than the theory predicts, changing the speed at which products are formed and also possibly changing which products are formed. Assumes that there are only 3 species involved in the transition state. This assumption leads to the idea that the only energy required for the reaction is the activation energy. However, in reality, different vibrational modes are possible for each of the species. Hence, the actual energies involved in the reaction are not just the activation energy.
(Fv611 (talk) 15:41, 17 May 2017 (BST) You don't mention the differences between TST predicted rates and experimental ones)
Exercise 2: F - H - H system
Classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved?
F + H2 is an exothermic reaction and H + HF is endothermic. This is because the H-F bond enthalpy value is higher than that of H-H.
(Fv611 (talk) 15:41, 17 May 2017 (BST) Correct, but you provide no justifications for your statements)
Locate the approximate position of the transition state

The reaction F + H2 is an exothermic reaction. According to the Hammond postulate, in an exothermic reaction, the transition state resembles the reactants rather than the products. As a result it can be seen that the transition state occurs when: AB (HF) is 1.80 and BC (HH) is 0.75.
Report the activation energy for both reactions
Potential Energy for the Transition State = -103.6kcal/mol
Potential Energy for the H-H / F = -103.4kcal/mol
Potential Energy for the H-F / H = -133.8 kcal/mol
Therefore,
Ea for F + H-H = 0.2 kcal/mol
Ea for H + H-F =30.4 kcal/mol
In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. How could this be confirmed experimentally?
The reaction F + H2 is exothermic. This means that the energy required to make the reaction go gets stored in the newly made F-H bond. To confirm this experimentally, the reverse reaction can be carried out and see whether the same amount of energy gets released or not.
(Fv611 (talk) 15:41, 17 May 2017 (BST) There is a confusion here on what exothermic vs endothermic reactions are. The reverse of an exothermic reaction is endothermic, and will not release energy. Also there is no discussion of the mechanism of energy release, nor of experimental procedures.)
Discuss how the distribution of energy between different modes (translation and vibration) affect the efficiency of the reaction, and how this is influenced by the position of the transition state
Based on the Hammonds Postulate, the exothermic reactions have early transition states. With the higher translational energy, the reactants can rearrange themselves to form the transition state more easily. Hence, the reaction efficiency is higher when there is more translational energy relative to vibrational energy.
(Fv611 (talk) 15:41, 17 May 2017 (BST) No discussion here. You should have provided examples. It's really a shame you didn't put the same amount effort in the second part of this exercise as you did in the first one.)