MRD:lz3717
===Molecular Reaction Dynamics: Applications to Triatomic systems===
Exercise 1: H + H2 system
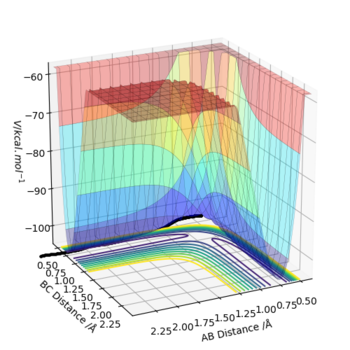
Q1:On a potential energy surface diagram, how is the transition state mathematically defined? How can the transition state be identified, and how can it be distinguished from a local minimum of the potential energy surface?
The transition state is the saddle point in this diagram, which is defined as the maximum on the minimum energy path linking reactants and the products.
Based on the mathematical expression, suppose there is a function f(x,y) with two different variables x and y.For a stationary point(x0,y0),the first derivatives of x and y must equal to zero.(fx=fy=0).
The second derivatives is illustrated as fxx and fyy.If a stationary point that fxxfyy-fxy2>0 and fxx>0,it is a local minimum point of this function.
If a stationary point that only satisfies fxxfyy-fxy2>0,it is stated as the saddle point.
(Good.) Cq3417 (talk) 00:13, 7 June 2019 (BST)
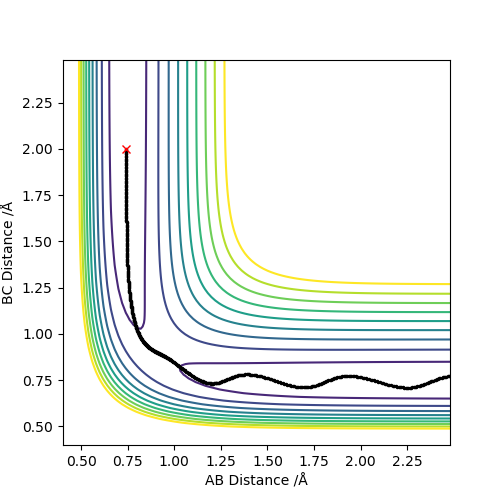
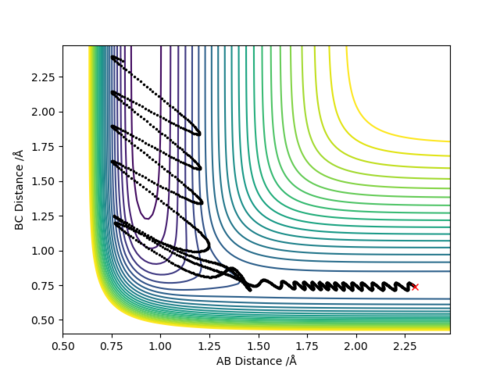
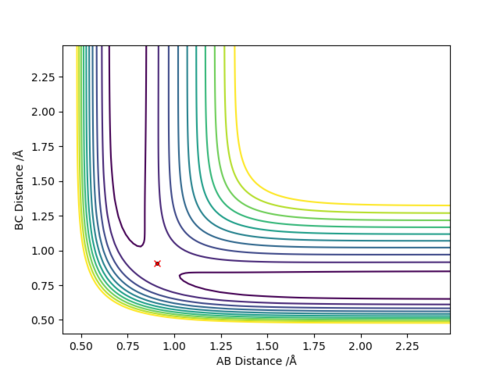
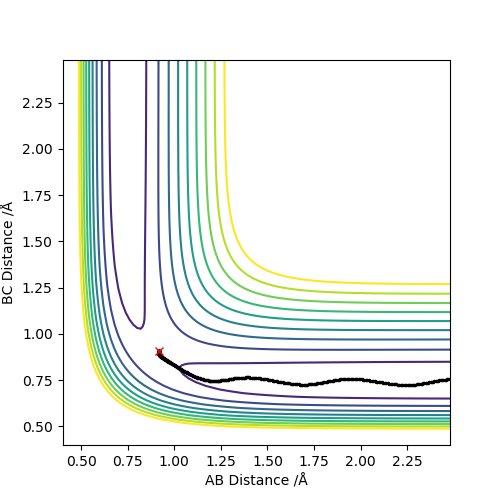
Q2:Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” plot for a relevant trajectory.
 |
 |
|---|
The best estimate of rts is 0.9077.As seen on the contour map, it is the saddle point of PES which illustrates the transition state. For the internuclear distance vs time graph,at rts,internuclear
distance does not change with time,which shows that the atoms stop moving when it is transition state,the potential energy reaches maximum and kinetic energy should be 0.
(Good illustration.) Cq3417 (talk) 00:13, 7 June 2019 (BST)
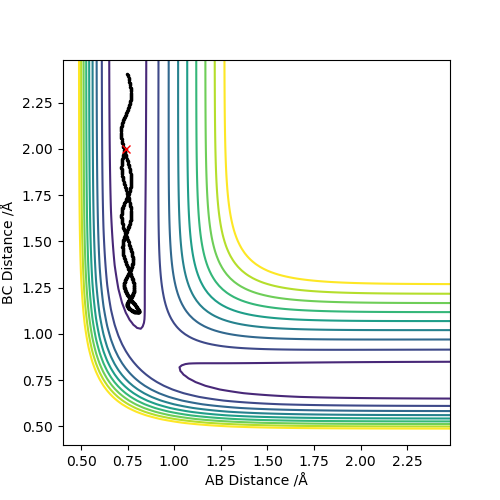
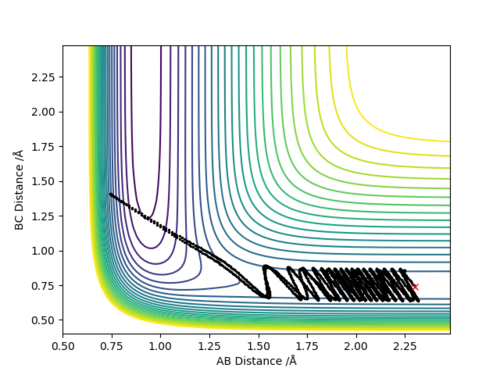
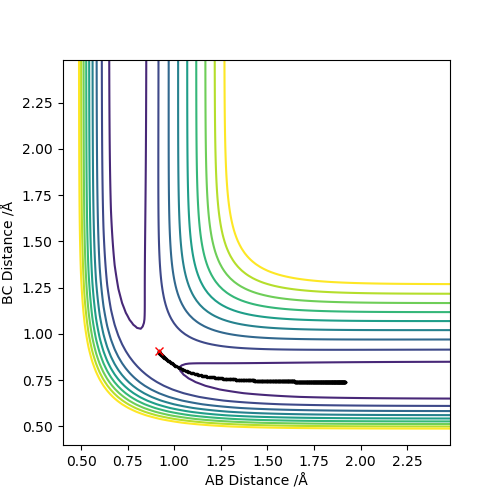
Q3:Comment on how the mep and the trajectory you just calculated differ.
Set the parameter r1=0.9177,r2=0.9077,p1=p2=0.
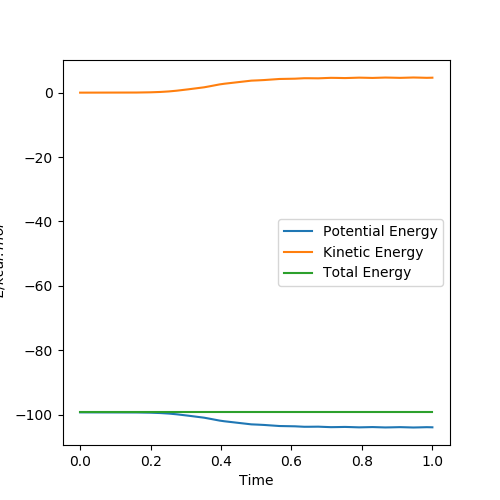
The difference to calculate reaction path by MEP and dynamic types are shown below:
 |
 |
|---|
It can be seen that in the MEP calculation,the molecule doesn't show any vibrations(velocity is set to be zero),while the molecule is vibrating in the dynamic calculation.
 |
 |
|---|
The MEP calculation shows the kinetic energy is always zero and the total energy is only potential energy,which means that the total energy is not conserved.However,the dynamic calculation shows conservation of total energy(kinetic energy increases while potential energy decreases).
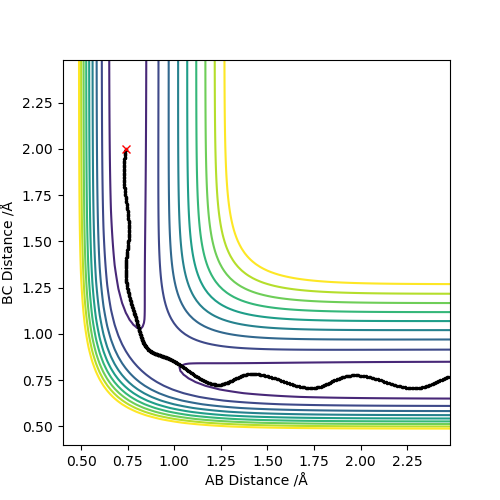
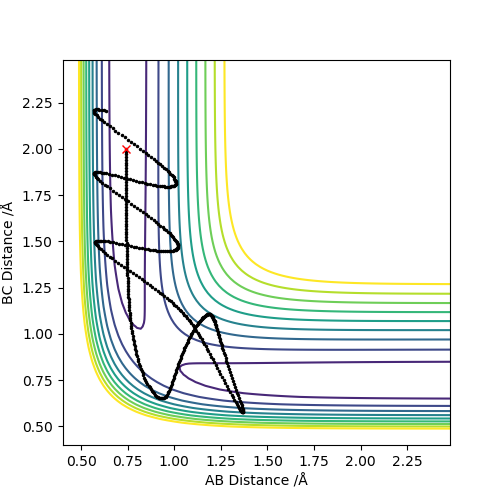
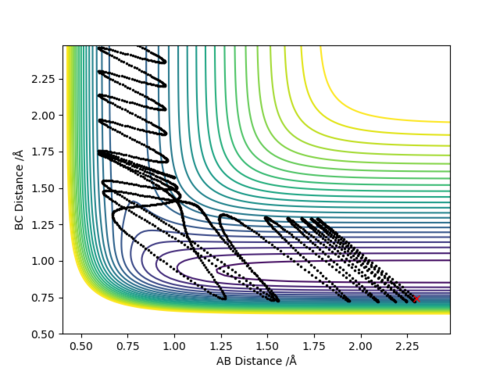
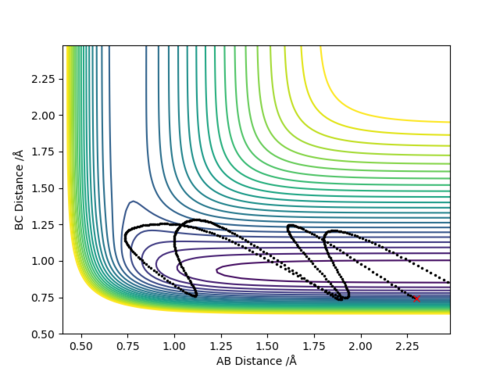
Q4:Complete the table above by adding the total energy, whether the trajectory is reactive or unreactive, and provide a plot of the trajectory and a small description for what happens along the trajectory. What can you conclude from the table?
Conclusion:In order to make the reaction happen,reactants have to gain enough kinetic energy to lead sufficient collision.Higher the kinetic energy,the more possible that the reaction will happen.However,high kinetic energy
cannot guarantee the successful reaction even if it passes the transition state,reactants may reform at this situation.
(Good discussion.) Cq3417 (talk) 00:13, 7 June 2019 (BST)
Q5:State what are the main assumptions of Transition State Theory. Given the results you have obtained, how will Transition State Theory predictions for reaction rate values compare with experimental values?
There are following three main assumptions of Transition State Theory: [1]
- ↑ Steinfeld J I, Francisco J S, Hase W L.Chemical Kinetics and Dynamics. 2nd ed. New Jersey: Prentice-Hall, Inc; 1998
1.Reactants are in constant equilibrium with the transition state structure.
2.The energy of the particles follow a Boltzmann distribution.
3.Once reactants become the transition state, the transition state structure does not collapse back to the reactants.
From the results obtained above,the product does will come back to reform the reactant even if it already crosses the transition state,which has a conflict with one of the assumptions.Therefore,the rate of the reaction in
reality is much slower than that theoretically because the product goes back to reform the reactant.
Exercise 2: F - H - H system
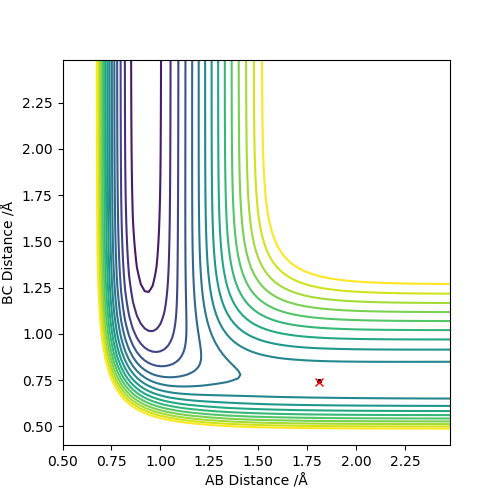
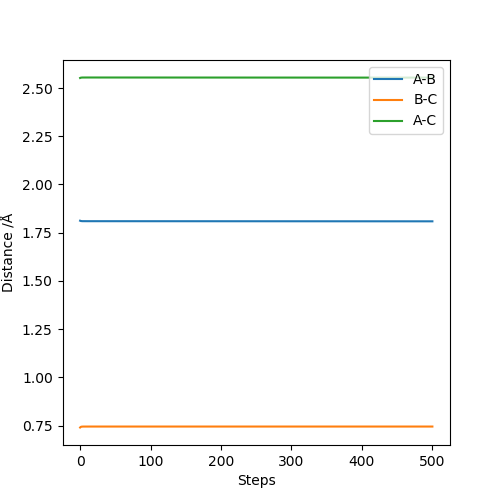
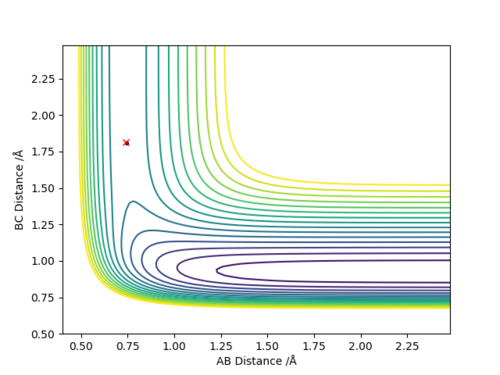
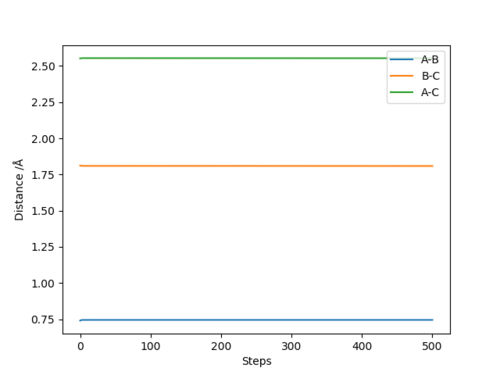
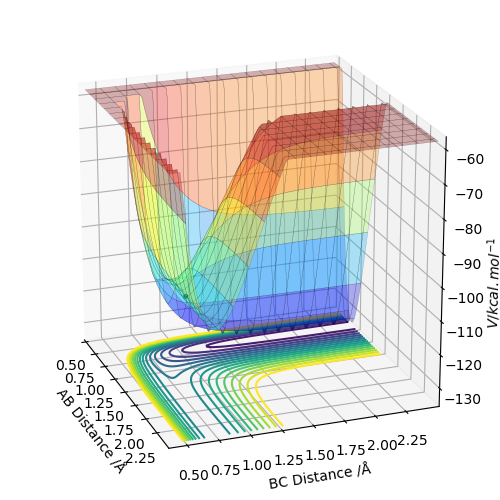
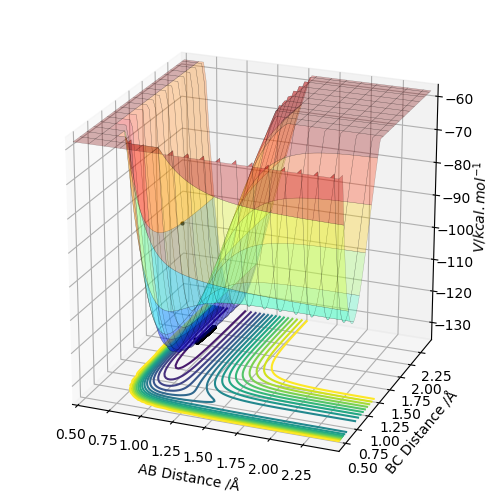
Q6:By the inspecting the potential energy surfaces,classify the F + H2 and H+HF reactions according to their energies(exothermic or endothermic).How does this relate to the bond strength of the chemical species involved?Locate the approximate position of the transition state.
 |
 |
|---|
For the F+H2→HF+H reaction,the position of transition state is located at HF=1.812 and HH=0.745.The reaction is exothermic because the energy needed for HF bond forming(-565KJ/mol) is larger than that for HH bond
breaking(+436KJ/mol).For the H+HF→F+H2 reaction,the position of transition state is located at HH=1.812 and HF=0.745.The reaction is endothermic because the energy needed for HH bond forming(-436KJ/mol) is lower than
that for HF bond breaking(+565KJ/mol).The F+H2→HF+H exothermic reaction shows that the H-F bond is stronger than H-H bond.
(Nice illustration.) Cq3417 (talk) 00:13, 7 June 2019 (BST)
Q7:Report the activation energy for both reactions.
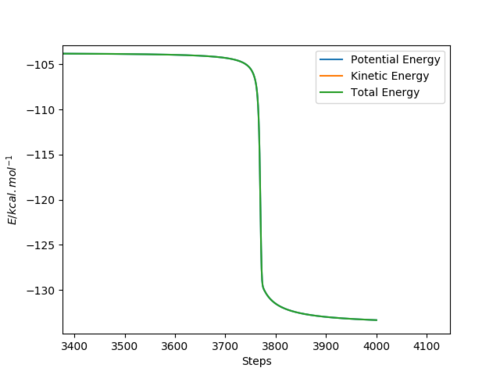
When the distance HF is changed to be 0.745,a structure neighbouring transition state is obtained.Because the distance HF is smaller than the real distance of HF in transition state,so the reaction tends to move towards reactants F and H2.As seen on the graph,the difference between highest energy and lowest energy is calculated to be the activation energy.
 |
 |
|---|
Overall:
The activation energy of F+H2 reaction is calculated to be +0.131kcal/mol.
The activation energy of H+HF reaction is calculated to be +31.21kcal/mol.
Q8:In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. Explain how this could be confirmed experimentally.
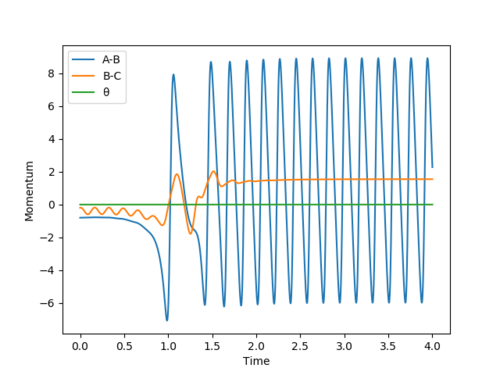
Because now the total energy is conserved,so the calculation type needs to be changed to dynamics.The initial conditions of F+H2 is set to be r1=1.90,r2=0.74,p1=-0.8,p2=-0.2.
 |
 |
 |
|---|
As it mentioned before,this F+H2 reaction is an exothermic reaction.After the reaction,there is an increase in kinetic energy and decrease in potential energy,which can be observed by the increase in temperature of the reaction.The total energy is still conserved because the excess kinetic energy transfers to the vibration energy of H-F bond,which can be proved by the fluctuated changing momentum of A-B bond in the momentum vs time plot.It means that the H-F bond is vibrating.This phenomenon can be observed from IR spectrum because H-F bond is IR active.
Q9:Discuss how the distribution of energy between different modes (translation and vibration) affect the efficiency of the reaction, and how this is influenced by the position of the transition state.
For F+H2 reaction,because it is a exothermic reaction,according to the Hammond postulate,the energy of transition state resembles the energy of reactants,so it has an early energy barrier.Thus,the translation energy
plays a more important role than vibration energy when crossing the energy barrier.
For H+HF reaction,because it is a endothermic reaction,according to the Hammond postulate,the energy of transition state resembles the energy of products,so it has an late energy barrier.Thus,the vibration energy
plays a more important role than translation energy when crossing the energy barrier.
(Good, but any reference?) Cq3417 (talk) 00:13, 7 June 2019 (BST)