MRD:ld2416
H + H2 System
On a potential energy surface diagram, how is the transition state mathematically defined? How can the transition state be identified, and how can it be distinguished from a local minimum of the potential energy surface?
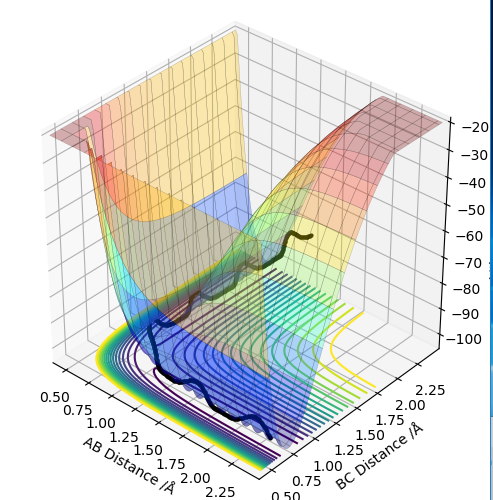
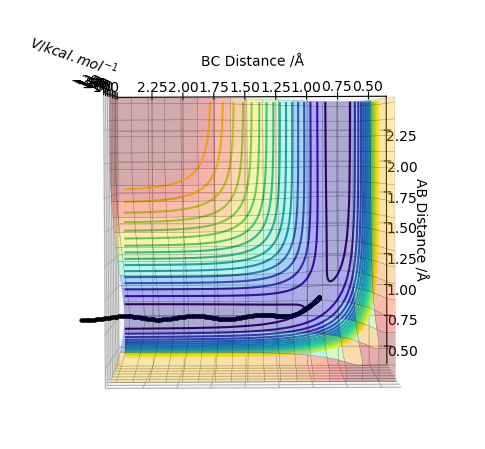
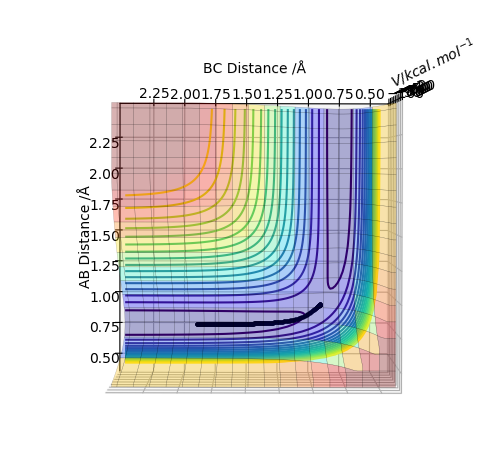
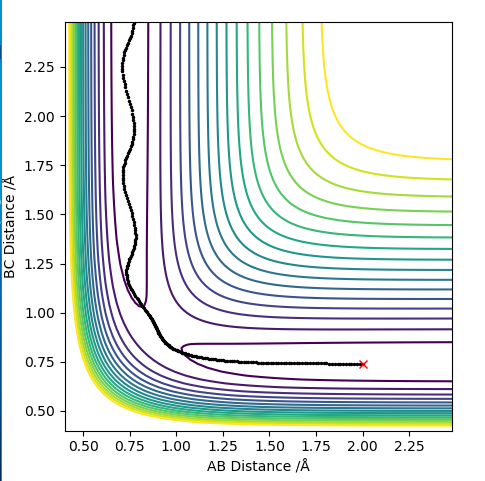
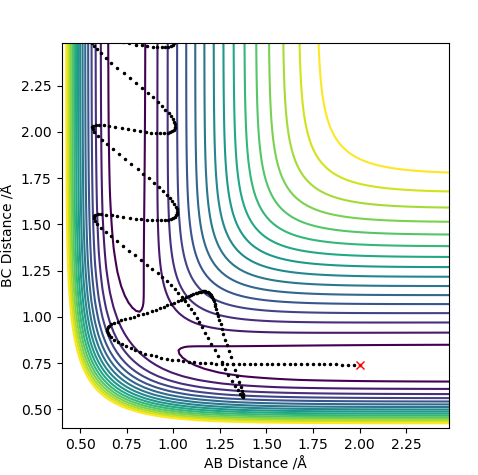
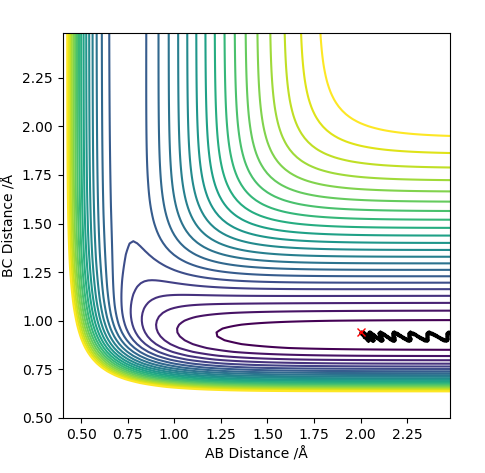
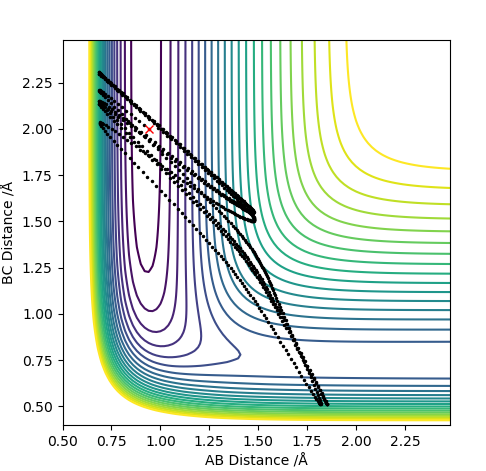
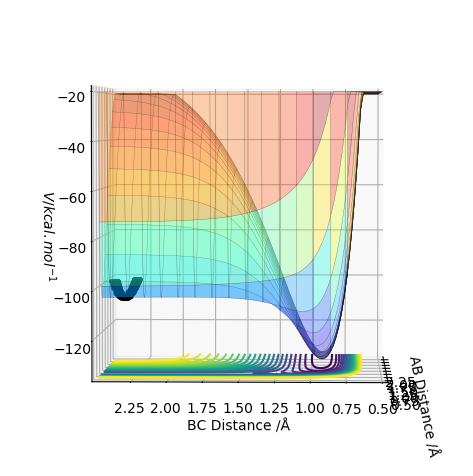
The transition state is when the H2 is equally distant to H1 and H3, for the contour plot this is approximately 0.93 A.
The transition state can be seen at the saddle point, where the transition state is a minimum for the frank condon plot for the reaction. The transition state is a maximum for the reaction trajectory. Mathematically, the TS has a second derivative greater than 0 in the Q1 direction (minimum) and TS has a second derivative less than 0 in the Q2 direction (maximum).
What do you mean by 'frank condon plot'? Maybe provide a reference... Fdp18 (talk) 10:48, 27 May 2019 (BST)
How are Q1 and Q2 defined? I know what you mean, but you cannot assume that for every reader. Also, is that the only criterion for a saddle point? Think back to curve sketching. Fdp18 (talk) 10:50, 27 May 2019 (BST)
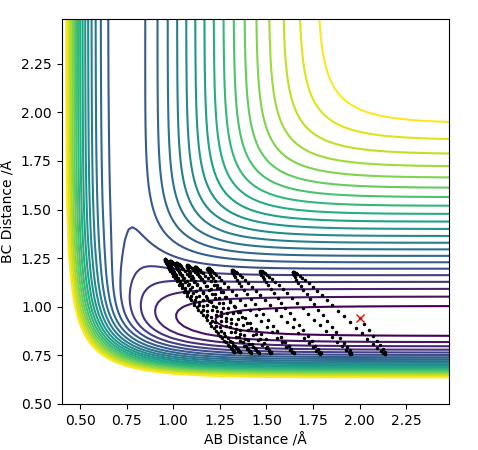
Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” plot for a relevant trajectory.
From the contour plot we see that the transition state occurs when the r1=r2. Therefore to estimate the transition state position distances AB must be equal to BC.
From this we can estimate the transition position from the contour plot:
This gives a value of 0.9075 A
Looking at this on a distance/time graph:
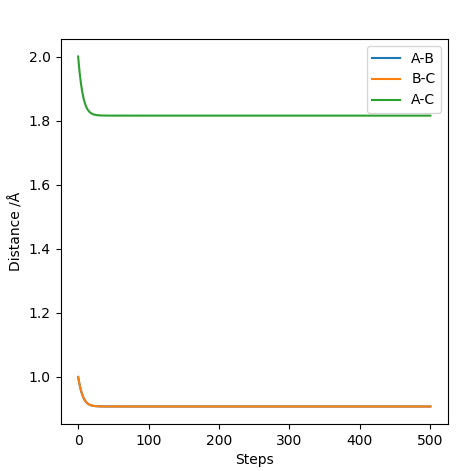
The momentum is set to 0 because the transition state is stationary and the distance between the 2 are equal.
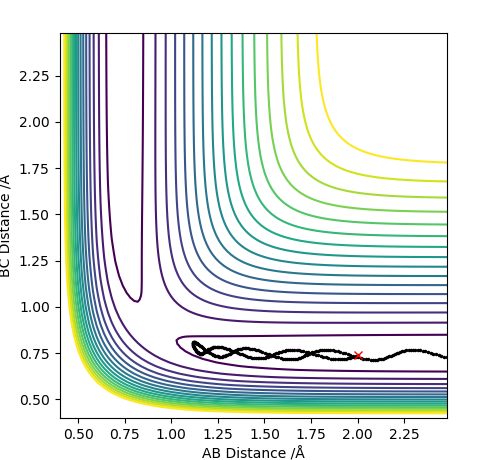
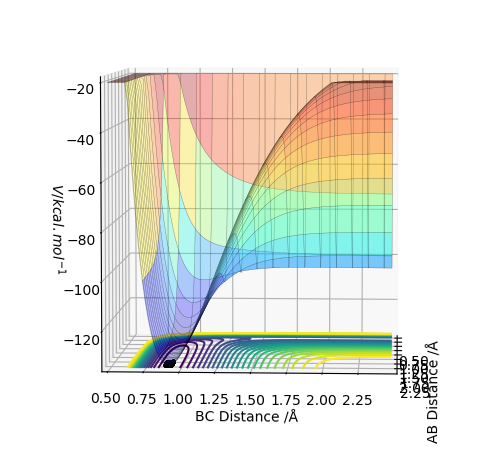
MEP surface diagram:
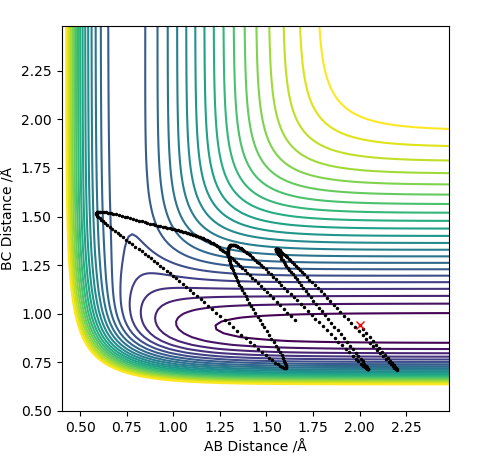
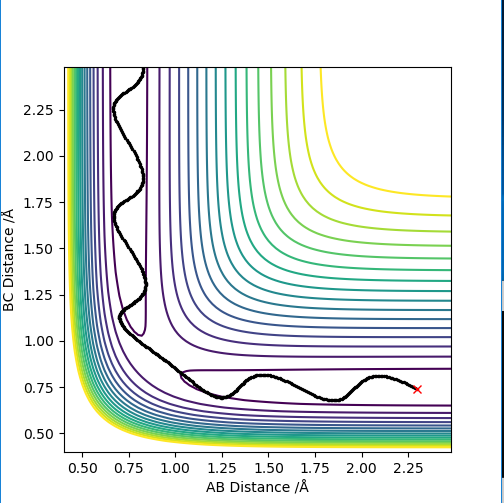
Differences can be seen in the length and oscillations in the reaction trajectory. For the minimum energy pathway (MEP) there is no oscillations as there it neglects the molecules vibration modes and it moves infinitely slowly. The line is shorter as the reaction coordinate isn't as long due to the reaction.
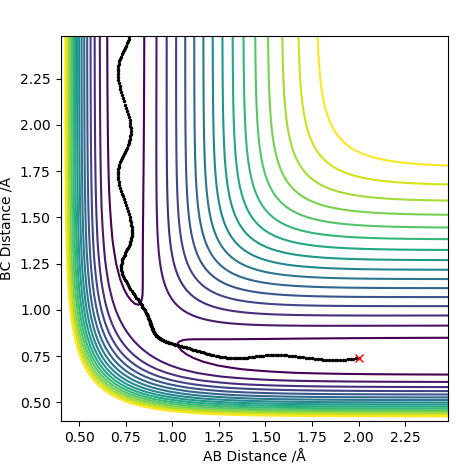
For the penultimate conditions, the reaction reaches the transition state yet its still falls back to the reactants despite the high momentum from the approaching H. The difference for the reaction to occur is the momentum to the approaching H to the molecule. It can be seen that the both the species in the reactants need the required momentum in order to reach transition state and to form the products.
I can't find the part where you state the main assumptions of TST. Fdp18 (talk) 10:53, 27 May 2019 (BST)
F-H-H system
H2 + F potential energy surface:
From the potential surface we see that the products; H2 + F are higher in energy than the product F-H this suggests that the F-H bond is stronger than the H-H bond. This reaction is exothermic. The reverse reaction must then be endothermic for H + HF
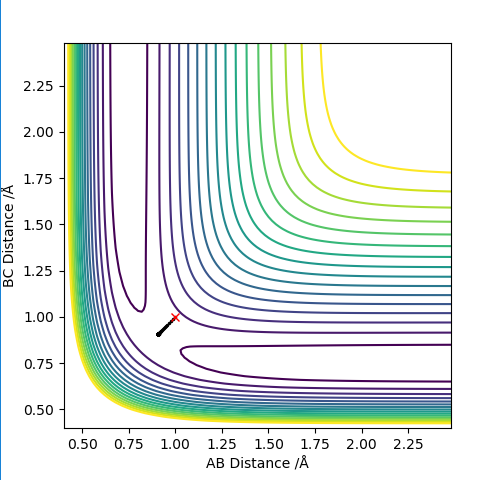
Transition state position H2 + F
To find the transition state, initial conditions were set and momentum is set to 0. From this it is clear that it isnt optimised as there are forces present between HH and F.
Setting HH distance to 0.744 and HF distance to 1.811, the forces are approximately 0 and so this is where the transition state exists.
Activation Energy H2 + F
Looking at the energy v time graph the difference between the total energy and the potential energy results in the activation
Activation energy = 104.019 - 103.749 = 0.270
What are the units here? Usually, atomic units (Hartree) are assumed when you don't give them. Fdp18 (talk) 10:56, 27 May 2019 (BST)
Transition state position HF + H
Transition state occurs at 0.74 A this resembles the H-H bond length in molecular H2
More than one bond distance is required to appropriately describe a three-body system (technically, two coordinates aren't sufficient either). Compare the values with your previous transition state - do you notice something? Fdp18 (talk) 10:58, 27 May 2019 (BST)
Activation E HF + H
The activation energy for this reaction can be calculated by the difference between the TS and the energy of the reactants HF + H

133-103 = 30
again, what are the units? Fdp18 (talk) 10:59, 27 May 2019 (BST)
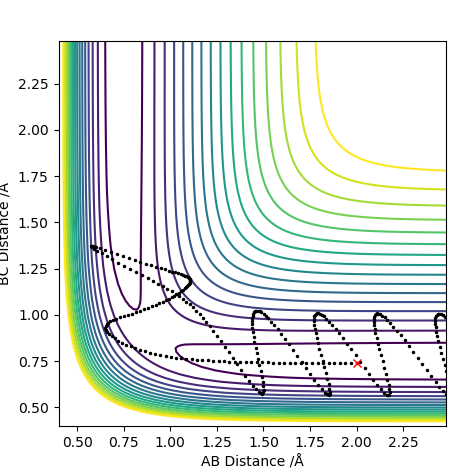
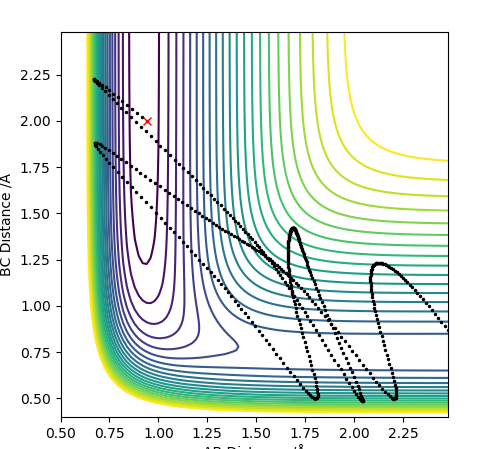
F + H2 reaction trajectory:
These are the initial conditions selected that gave a reaction trajectory
The momentum v time plot shows B-C (H-H) vibrating as F has little momentum. Once reaction happens, BC momentum stops oscillating and AB (HF) starts oscillating showing the conservation of energy as the vibration transfers from HH to HF. The mechanism of release:
rHH=0.74, rHF=2 and pHF = -0.5 for the following table. The pHH ranges from -3 and 3 to see if a reaction occurs with these initial conditions.
| pHH | Reaction? | Contour Plot! |
|---|---|---|
| 3.0 | No | 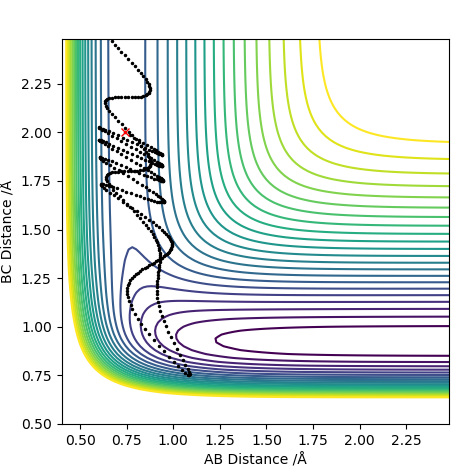
|
| 2.0 | No | 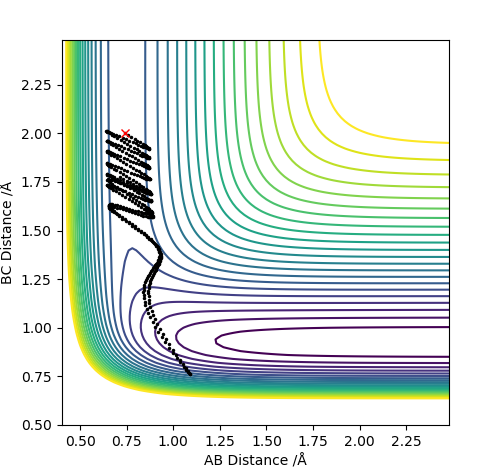
|
| 1.0 | No | 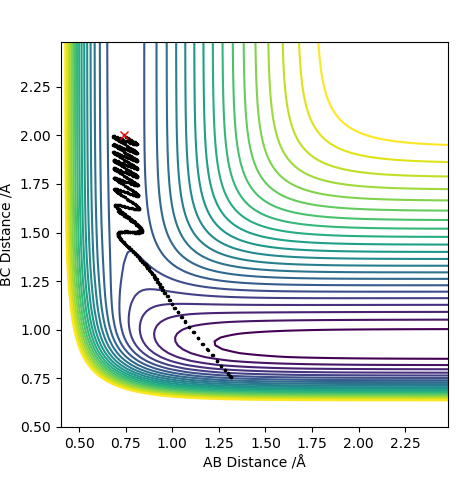
|
| 0 | No | 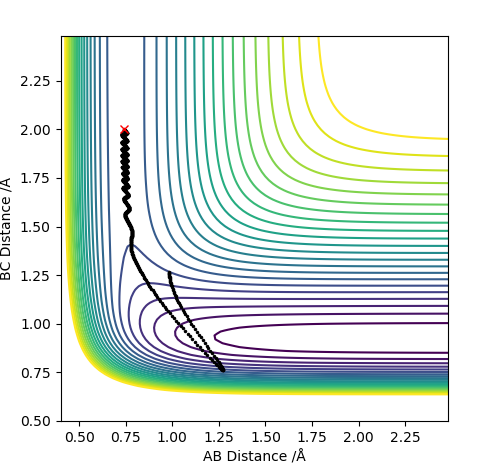
|
| -1.0 | No | 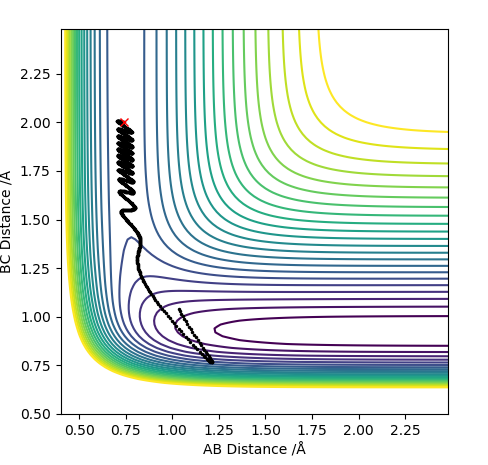
|
| -2.0 | Yes | 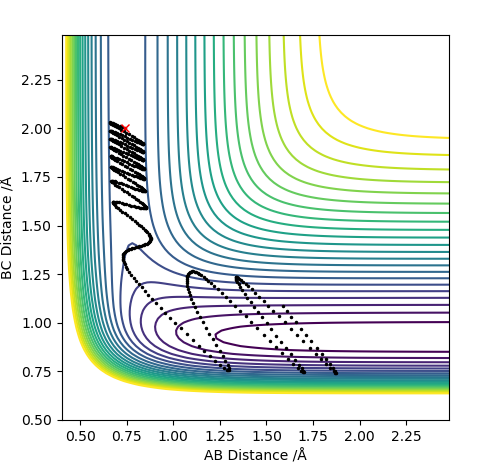
|
| -3.0 | No | 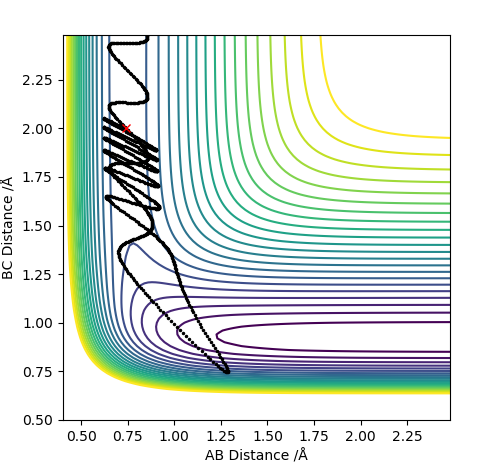
|
For F having momentum -0.5, reaction only occurs when the HH bond has -2 momentum. For reaction to complete is not enough to have a large energy in the system. Each both H2 and F require specific energy in order to react with each other therefore changing either energy values will require the other species to have a complimentary energy.
I appreciate the effort. However, most of these trajectories stop a bit early, where it is not yet clear whether the reaction will go to completion or not. Fdp18 (talk) 11:03, 27 May 2019 (BST)
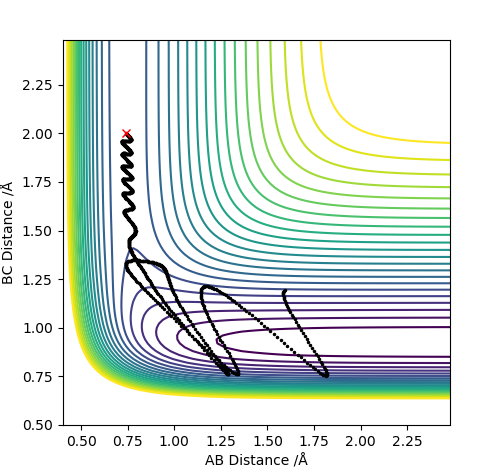
H + HF reaction trajectory:
Setting the conditions: r1 = 0.94, r2 = 2
Polanyi's empirical rules state there is a linear relationship between the activation energy and the position of the transition state. For H + HF there is a late transition state and therefore a higher Ea is needed compared to H2 + F. For late transition states the reactants require high vibration energy, which can be changed by altering the momentum. For other high energy reactions for HF + H the reaction was unsuccessful due to the composition of momentum being vibrational and translation energy. For the unsuccessful reactions the composition of vibrational and translation energy did not have enought vibrational energy for the reaction to go past the TS. On the other hand early stage TS need more translation energy.
And how could these mechanisms be investigated experimentally? Fdp18 (talk) 11:05, 27 May 2019 (BST)
Please get in the habit of providing references, especially for external content like Polanyi's rule. Fdp18 (talk) 11:06, 27 May 2019 (BST)