MRD:kt3817
Problem 1
1. On a potential energy surface diagram, how is the transition state mathematically defined?
How can the transition state be identified, and how can it be distinguished from a local minimum of the potential energy surface?
Transition state is mathematically defined as aV(ri)/ari=0 or that it is the point where the gradient of the potential is zero.
Transition state can be identified by starting trajectories close to the transition state and see if they go towards reactants or the products. Transition state is the maximum of the minimum energy path so it is not a local minimum. If the point is a local minimum and not a transition state then the trajectory, when started close to the minimum, will always go to that minimum, whereas trajectory started on either side of the transition state will go to either reactants or products. Second derivative negative for local minimum, positive for transition state which is a maximum.
What is that function? This is not clear. The partial derivative is zero at transition state. And its the second partial derivative, which is also negative at the local maximum. Sf3014 (talk) 22:56, 4 June 2019 (BST)
2. Estimated position of a transition state is r(ts)=0.9078.
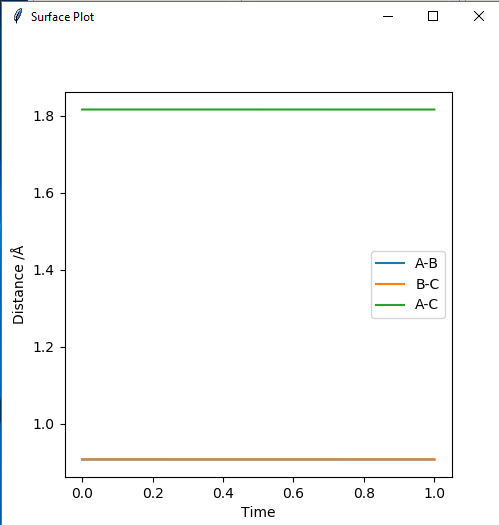 Good, but how did you get this value?. Sf3014 (talk) 22:57, 4 June 2019 (BST)
Good, but how did you get this value?. Sf3014 (talk) 22:57, 4 June 2019 (BST)
3. How do the mep and dynamics trajectory differ?
The minimum energy path is a straight line whereas the trajectory calculated with the dynamics method oscillates (vibrations are visible). What do you conclude from this observation? What are these observations from? You should include the relevant graphs and refer to them. Sf3014 (talk) 23:00, 4 June 2019 (BST)
4. Table
Good observations in graph but what do you concluded from this?. Sf3014 (talk) 23:12, 4 June 2019 (BST)
5. State what are the main assumptions of Transition State Theory. Given the results you have obtained, how will Transition State Theory predictions for reaction rate values compare with experimental values?
Transition state theory assumes that particles move according to classical mechanics and that if there is not enough kinetic energy for particles to react, they will not react. Transition state is a saddle point on a potential energy surface and a maximum of the minimum energy path between reactants and products. In a transition state, reactants and products are in an equilibrium state between the two states and can be moved to either reactants or products with a small change in geometry.
Transition State Theory assumes that when particles collide with an enough energy to react, they will react regardless of which kind of kinetic energy it is. Results showed, however, that when pathway is reactive with certain amount of vibrational and translational kinetic energy, when the amount of vibrational energy, for example, is decreased and the amount of translational energy is increased so that the overall energy remains the same, it does not mean that the second reaction path is also reactive. Good but how does transition state theory (TST) compare to you results? Refer to examples in your table. And sorry, your final sentence is very confusing, I don't understand your point. Also, how does TST compare to the experimental reaction rate? And where did you get this information on TST? reference, please. Sf3014 (talk) 23:12, 4 June 2019 (BST)
Problem 2
1. By inspecting the potential energy surfaces, classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved?
F + H2 reaction is exothermic, H + HF reaction is endothermic. H-F bond is stronger than H-H bond so when F + HH reaction takes place, the weaker bond is broken and stronger bond is formed which makes reaction exothermic, with H + HF reaction, stronger bond is broken and reaction is endothermic.
How is this seen in your potential energy surface (PES)? Show PES and refer to it. Also, it would be better if you included reported values of the bond energies for HF and H2 in your description. Sf3014 (talk) 23:15, 4 June 2019 (BST)
2. Locate the approximate position of a transition state.
When atom A is F and atoms B and C are H atoms:
AB distance is 1.81
BC distance is 0.745
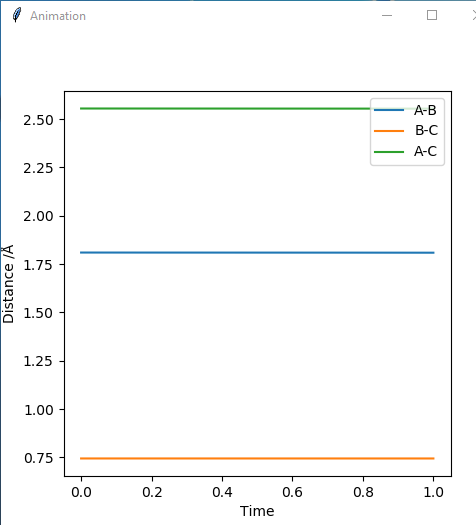 Good but how did you get to this value?. Sf3014 (talk) 23:17, 4 June 2019 (BST)
Good but how did you get to this value?. Sf3014 (talk) 23:17, 4 June 2019 (BST)
3. Activation energies
Activation energy can be calculated by taking the energy of the transition state and substracting the energy of the respective species, either H2 + F or HF + H. The energies of the molecules can be found by giving the distance of the other atom to be infinetely large.
Transition state energy is -103.752
For F + H2: 103.752- 33.154 = 70.598
For H + HF: 104.190- 103.752 = 0.348 Good but I'm assuming that the "respective species" are the reactants? Also, you can show this with your energy vs time graph which will be useful to show your workings because the former activation energy is quite large. Sf3014 (talk) 23:21, 4 June 2019 (BST)
4.
Conditions:
r1 = 1.0
r2 = 2.0
p1 = -2.5
p2 = -5.0
First, H2 molecule approaches the F atom and H-H atoms are vibrating. As the H atom closest to the F atom reaches the F atom, H-H bond will get weaker and simultaneously, H-F bond is formed. The vibration starts in H-F molecule and the other H atom will distance from HF.
From momenta vs time plot, similar thing can be seen as first, there is vibration between the two H atoms as they approach F atom and when the bond breaks, momentum remains constant. For H-F bond, momentum is constant while H2 molecule approaches it, then drops and increases again as the bond forms and the vibration pattern starts.
The overall momentum has increased throughout the reaction therefore there is more kinetic energy and less potential energy in the system. The reaction has an early transition state.
Are you describing the conservation of energy? This is not clear. Sf3014 (talk) 23:28, 4 June 2019 (BST)
5.
Both translational and vibrational energy affect the efficiency of the reaction, however, according to examples done above about H-H-H system, translational energy seems to have a bit larger role in whether or not the reaction is efficient. For example, when going from first to second row of the table, translational energy is decreased and vibrational energy is increased by the same amount, however, the reaction only happens during the first but not during the second case.
Transition state is always higher in energy than the reactant or product state. If the reaction is exothermic, it has an early transition state and when reaction is endothermic, it has a late transition state. This means that in case of an exothermic reaction, translational energy is converted into vibrational earlier than in the case of an endothermic reaction which has a late transition state. I think there is some confusion here. You were meant to relate Polany's rules to your H2 + F <-> H + HF reaction. Sf3014 (talk) 23:28, 4 June 2019 (BST)





