MRD:jwy17mrd
Molecular Reaction Dynamics
Transition State
"On a potential energy surface diagram, how is the transition state mathematically defined?How can the transition state be identified, and how can it be distinguished from a local minimum of the potential energy surface?"
To define a transition state, we shall consider 2 new 2-D basis vectors orthoganol to each other, q1 and q2, with q2 parallel to the trajectory of the reaction. These 2 basis vectors are diagonal to the existing basis vectors, which are r1 and r2. The transition state would be at the saddle point of the potential energy surface, where the criteria are as follows:
- The first order partial derivative of the potential energy surface w.r.t q1 and q2 are zero.
- The second order partial derivative of the potential energy surface w.r.t q1 is positive and negative w.r.t to q2.
It is important that both criteria to be fulfilled so that the point on the potential energy surface corresponds to the transition state.
A local minimum, while fulfill the first criteria, falls short to fulfill the second because the second order partial derivative of the potential energy surface w.r.t q1 and q2 is positive.
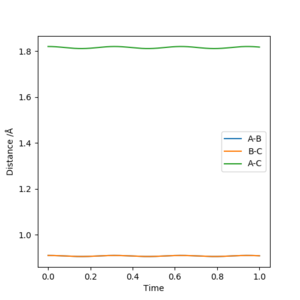
Trasition state of H2 + H System
"Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” plot for a relevant trajectory"
At the transition state, where hydrogens A and C are equidistant to the centre hydrogen B, would not oscillate if they have no momenta and do not posses excess potential energy that can be converted to kinetic energy. At 0.91 Angstrom, the Internuclear Distance Vs Time plot of the hydrogen atoms with 0 initial momenta showed a very minute oscillation. Therefore, by inspection (~0.8 Angstrom) and trial and error, the rts is 0.91 Angstrom.
(I think you can report better result for transition state with the aid of contour plot.) Cq3417 (talk) 15:31, 15 May 2019 (BST)
Trajectory of r1 = rts+δ, r2 = rts
"Comment on how the mep and the trajectory you just calculated differ." (show the potential energy surface top view dynamics vs mep)
The mep shows a non-oscilating trajectory, as it only displays the the path with the least potential energy. Realistically, the offset of the position of one of the hydrogens puts it on a negative gradient, moving the reaction off the ridge. The vibration arises the the difference between the equilibrium position of the product/reactant and the transition state position. As the equilibrium distance between the 2 hydrogens shift from the transition state position to that of hydrogen molecules, the hydrogen atoms find themselves at a position above the equilibrium. The potential energy can be converted to kinetic energy and vibrate, with inertia keeping it in motion.
Reactivity
"Complete the table above by adding the total energy, whether the trajectory is reactive or unreactive, and provide a plot of the trajectory and a small description for what happens along the trajectory. What can you conclude from the table?"
| P1 AB | P2 BC | Etot (kJ/mol) | Reaction? | Description of the dynamics |
|---|---|---|---|---|
| -1.25 | -2.5 | -99.018 | Yes | The molecule does not vibrate much initally and vibrates after passing through the transition state |
| -1.5 | -2.0 | -100.456 | No | The molecule vibrates but does not overcome the activation barrier and does not form a new molecule. |
| -1.5 | -2.5 | -98.956 | Yes | The molecule and new molecule both vibrates. |
| -2.5 | -5.0 | -84.956 | No | A new molecule was transient, as the molecules breaks to form a new molecule, but reverts back to the orginal molecule. |
| -2.5 | -5.2 | -83.416 | Yes | The new molecule reverts back to the orginal molecule once and then back to the new molecule. |
| P1 AB | P2 BC | Reaction? | Reaction trajectories |
|---|---|---|---|
| -1.25 | -2.5 | Yes | 
|
| -1.5 | -2.0 | No | 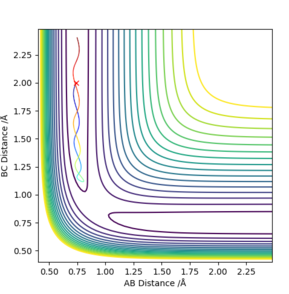
|
| -1.5 | -2.5 | Yes | 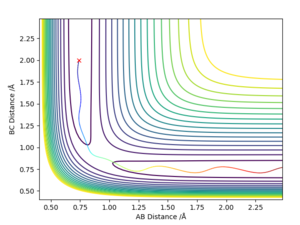
|
| -2.5 | -5.0 | No | 
|
| -2.5 | -5.2 | Yes | 
|
The amount of total energy the system does not indicate whether the reaction is reactive or not. Despite having the enough energy to overcome the activation energy barrier, the reactivity of the reaction is not guaranteed.
Transition state Theory
"State what are the main assumptions of Transition State Theory. Given the results you have obtained, how will transition State Theory predictions for reaction rate values compare with experimental values?
In Transition State Theory, it is assumed that:
1.The reactants and transition state follows a Boltzzman Distribution.
In the cases where for rapid reactions, the transition state may disappear rapidly, causing its concentration to be less than expected in the solution. This assumption overestimates the experimental rate value, as the transition state concentration is more than reality.
2. The reaction tranjectory only crosses the transition barrier once to form the product.
This is not true, as shown in the reactivity section, that can re-cross the barrier to form the reactant or re-cross in the direction of the products twice. This assumption also then overestimates the rate value because the actual value involves multiple re-crossing until it forms the reactants or products, hence less than the prediction.
(I think a citation of assumption is required here.) Cq3417 (talk) 15:31, 15 May 2019 (BST)
F-H-H System
Transition State of F-H-H System
"By inspecting the potential energy surfaces, classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved?
Locate the approximate position of the transition state."
By inspection, the reaction is exothermic, as the H + HF part of the potential energy surface miinium is lower than the F + H2. This means that HF has a much strong bond strength than the H2 speci
(It seems that something is missing here.) Cq3417 (talk) 15:31, 15 May 2019 (BST)
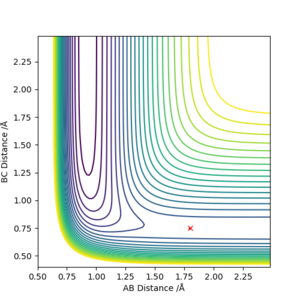
To find the transition state. The equilibrium position of the H2 are first estimated (~0.74 Angstrom). then by trial and error, the F-H position was adjusted until the trajectory of the reaction is not obvious (~ 1.82 Angstrom). The transition state fits the Hammond postulate, as it is a exothermic reaction and the transition state resembles a H2 molecule.
Activation energy
"Report the activation energy for both reactions"
H + HF to F + H2 : -103. 777 - (-133.269) = 30.0kcal/mol
F + H2 to H + HF: -103.738 - (-103.983) = 0.3 kcal/mol
*Values of the total energy of both intial and final states are measured using the built in ruler


Energy Dissipation
"In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. Explain how this could be confirmed experimentally."
As the HF molecule is formed, the potential surface minimum drops dramamtically. As the total energy is conserved:
- The HF may find itself at a much higher vibrational state. The vibrational energy can be transfered to the H atom as kinetic energy.
- The reaction may directly result in a HF in a ground-state vibrational mode and the H atom realesed gains kinetic energy.
As the kinetic energy of the particles increase, so will the temperature, as they are proportional to each other. Therefore, as we perform the reaction, an increase in temperature or pressure would be observed.
(I think you should include more experimental data to support your discussion.) Cq3417 (talk) 15:31, 15 May 2019 (BST)
Polanyi's Rule[1]
"Discuss how the distribution of energy between different modes (translation and vibration) affect the efficiency of the reaction, and how this is influenced by the position of the transition state."
Early Barrier
An early barrier is where the transition state would be on the PES when forming HF + H from F + H2 (Before turning the corner). A translational energy would be more efficient in carrying the trajectory over the transition state. The translational and vibrational energy requires to form the product is a balancing task, given too much of either can cause the tranjectory to 'bounce' back to the reactant.
Late Barrier
A late barrier is where the transition state would be on the PES when forming HF + H from F + H2 (After turning the corner). To access the transition state the HF molecule has to have a massive vibrational energy (large momentum). As with the early barrier, translational energy cannot be ignored and the right amount of translational energy is required still to reach the transition state.
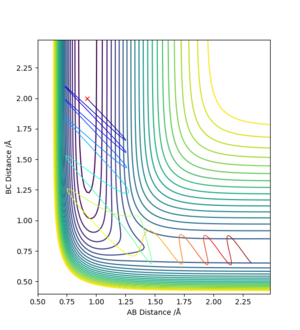
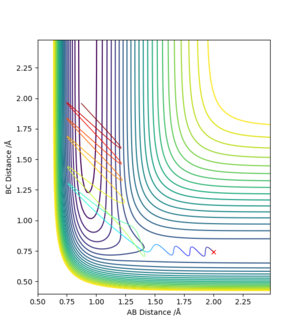
Overall, I think you can improve more if include more expeirmental data.Cq3417 (talk) 15:31, 15 May 2019 (BST)
- ↑ L. Arnaut, S. Formosinho and H. Burrows, Chemical Kinetics From melecular Structure to Chemical Reactivity, Elsevier, Oxford, 2007
