MRD:js2016
H + H2 system
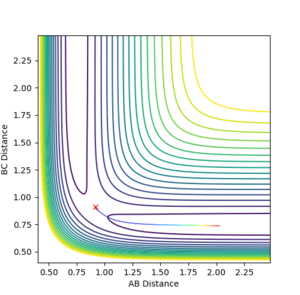
Considering components of the transition state
At a minimum or a transition structure, the gradient of the potential energy surface is zero in both orthogonal directions. However at the saddle point (transition structure), the minimum lies in one direction diagonal to r1 and r2 and the transition state lies orthogonal to the minimum. In order to distinguish between both, the second derivative can be used
Ng611 (talk) 12:31, 8 May 2018 (BST)A brief introduction before this section would have been useful.
Transition state position
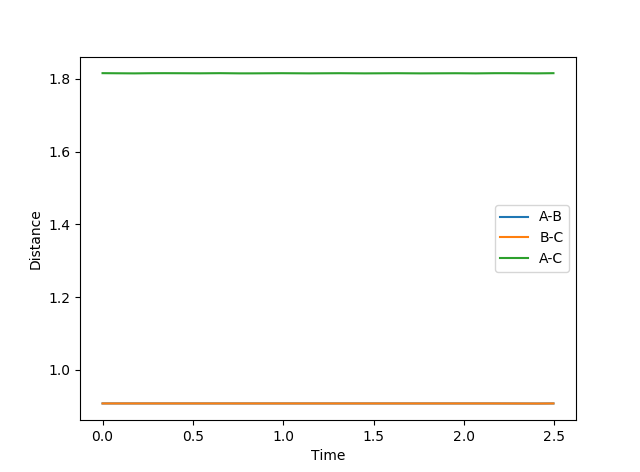
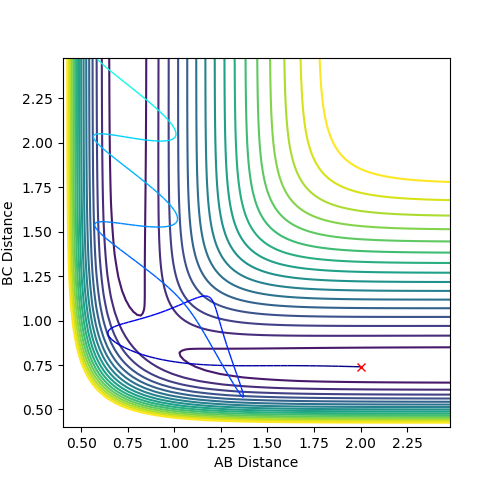
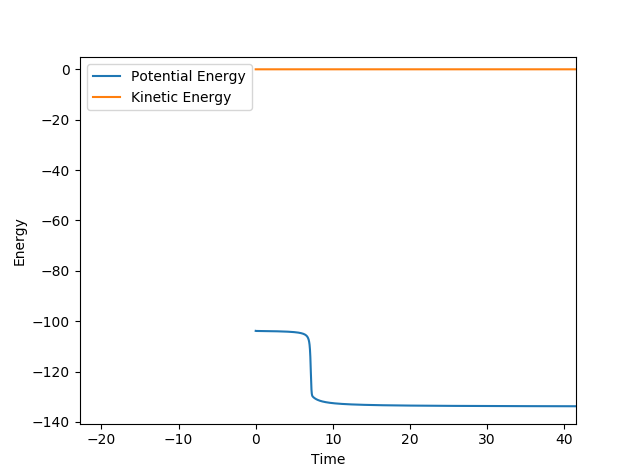
From the graph below of internuclear distances against time, there is negligible change in distance between the atoms when rts = r1 = r2 which shows that the transition state has been reached.
rts=0.9077 Å
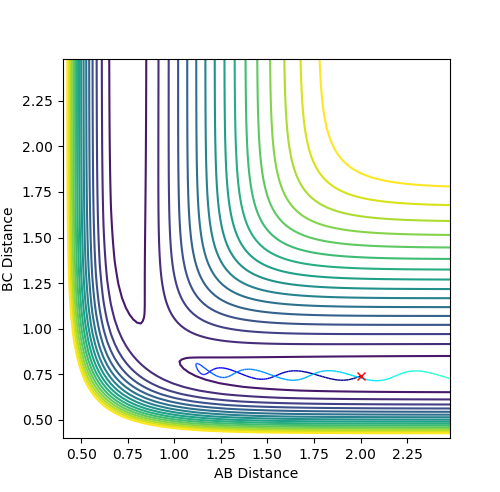
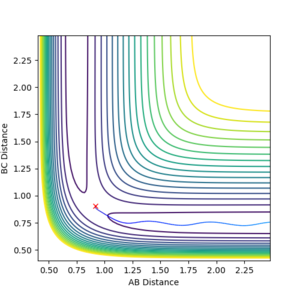
Trajectories from r1 = rts+δ, r2 = rts
When considering trajectories just off the transition state, the reaction path with the MEP calculation type shows a gradual change in the interatomic distance in the product molecule. However using the Dynamics calculation type follows a similar path but retains a more realistic version of the diatomic molecule vibrating as it leaves the transition state.
Transition State Theory
The main assumptions of Transition State Theory are that the atomic nuclei being considered behave according to classical mechanics. A quantum mechanical approach as well would take into account the possibility of reactants "tunneling" to the products, which increases the lower the activation energy. Also, the theory assumes that the reactants will pass over the lowest energy saddle point on the potential energy surface. At high temperatures, the higher energy vibrational modes are now accessible and the motion of these molecules is difficult to predict which leads to transition states forming further away from the lowest saddle point. This results in the products not actually forming and the system reverts back to the reactants. Transition State Theory predictions for reaction rate values will differ with experimental values in that TST at high temperatures for some reactions will predict a higher reaction rate than experimental values.
Ng611 (talk) 12:34, 8 May 2018 (BST)Good explanation. However, some additional discussion relating it to the results of your simulations would have improved it further.
H-H-F system
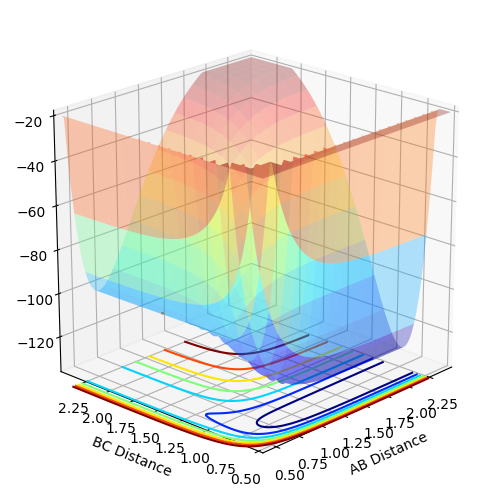
F+H2 is an exothermic reaction whereas H + HF is an endothermic reaction. This illustrates that the H-F bond is stronger than the H-H bond since the energy released from the formation of HF is greater than the energy released from the formation of H2. The potential energy surface of the H-H-F system is shown below where the products H + HF lie in the energy well where distance BC is constant and the products H2 + F lie in the energy well where distance AB is constant.
Transition state position is where ABC is HHF and distance AB = 0.744 Å and distance BC = 1.811 Å
Ng611 (talk) 12:40, 8 May 2018 (BST) How was this found?
The activation energy for H + HF = 29.779 kcal/mol. The activation energy for F + H2 = 0.263 kcal/mol
Ng611 (talk) 12:39, 8 May 2018 (BST) What do these two figures represent? A few lines explaining what they show you would have been useful.
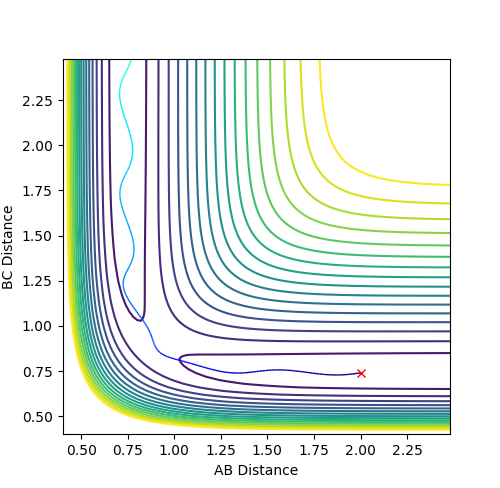
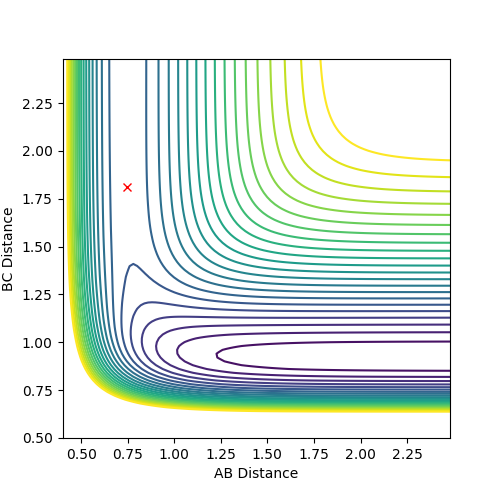
Reaction dynamics for F + H2
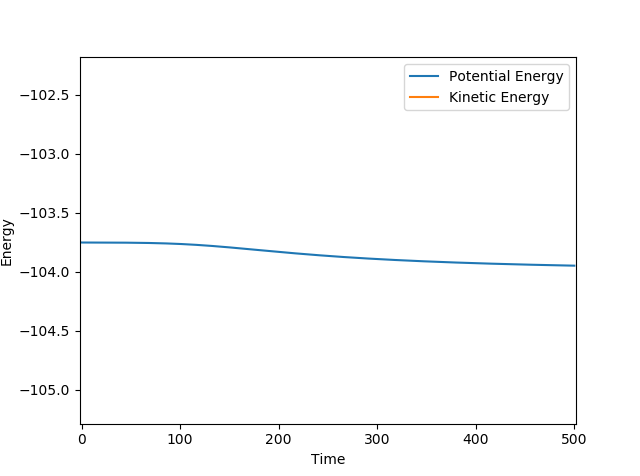
Initial conditions set are r2 = AB = 0.744 Å and r1 = BC = 2.25 Å, where p1 = -0.67 and p2 = 0. This produced a reactive trajectory. Energy is conserved by the significant vibrations of the product molecule (HF) after the reaction where the momentum of the HF molecule oscillates. This could be confirmed experimentally by measuring for an increase of temperature since the increased kinetic energy of the product HF molecules would be released and drive the temperature up.
Polanyi's rules state the vibrational energy is more significant in reactions where the transition state resembles the products and the translational energy is more significant in reactions where the transition state resembles the reactants. [1]
- ↑ 1 Z. Zhang, Y. Zhou, D. H. Zhang, G. Czakó and J. M. Bowman, J. Phys. Chem. Lett., 2012, 3, 3416–3419.
However, not every reaction follows these rules. In F + H2 where the transition state resembles the reactants, the H2 molecule moving with high translational energy towards the F atom is favored but the reaction still proceeds if the H2 molecule approached the F atom slowly but vibrating with high energy.
Ng611 (talk) 12:44, 8 May 2018 (BST) This section is severely lacking in content. You needed to simulate a range of trajectories for both the H2+F→HF+H and the HF+H→H2+F reactions for a range of internuclear momenta. Using only 1 trajectory is not enough to allow you to draw any conclusions.