MRD:jc2916
H + H2 System
Three H atoms system. The distance between A-B is r1, and distance between B-C is r2.
Dynamics from Transition State Region
When r1 is changing , the potential energy varies. At a certain r1 value, the potential energy is at its minimum, so the gradient vanishes and ∂V(r1)/∂r1=0. Because it is the minimum point of the V(r1), so the secondary derivative ∂V2(q2)/∂q22>0. The same can be applied to r2.
However, the transition state is the local maximum on the minimum energy pathway, which means it is the energy minima of V(q1), but energy maxima of V(q2). Therefore, ∂V2(q1)/∂q12<0, and ∂V1(q1)/∂q11>0. So transition state is the cross point of two curves, and if we adjust r2 by a small amount to in the direction of the products, it will roll towards the product.
Trajectories from r1 = r2
Jas213 (talk) 19:09, 28 May 2018 (BST) How did you find the TS?
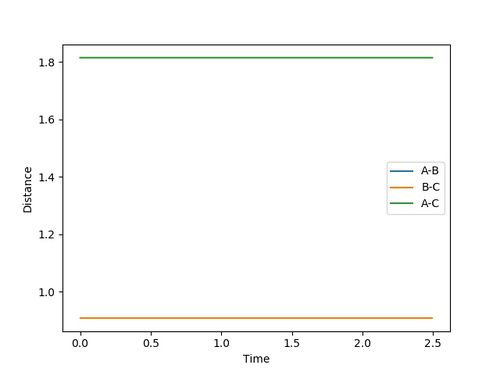
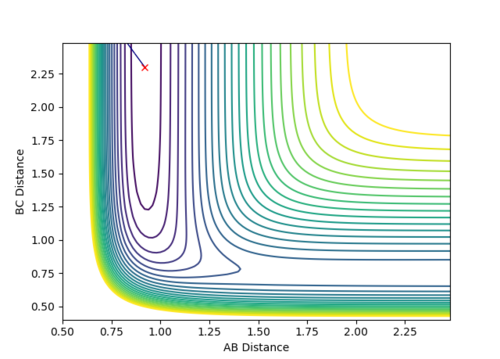
The transition state distance is estimated to be: rts = 0.9077500
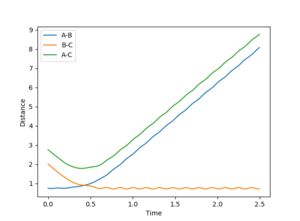
Because the trajectory starts at transition state, there is no initial momentum. The distance between three atoms, r1 and r2 are the same, and will stay the same without rolling to any other directions. Therefore, on the Internuclear Distances vs Time plot, distances between A-B, r1, and B-C, r2, stay constant at 0.9077500, and distance between A-C, which is double the length of rts, stays constant at 1.8155.
Trajectories from r1 = rts+δ, r2 = rts
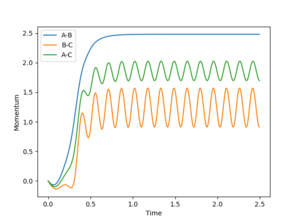
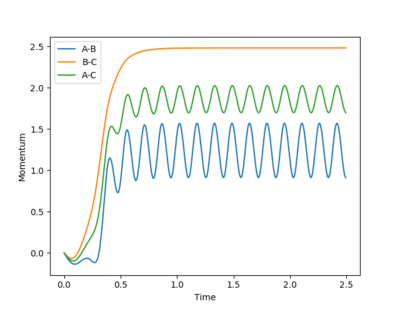
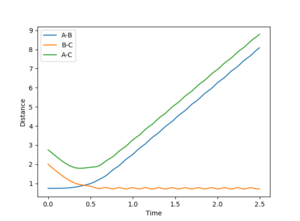
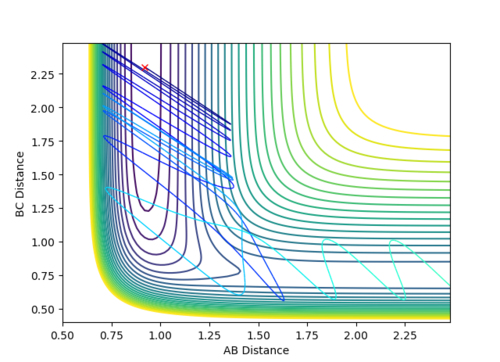
When r1 is 0.01 higher than rts, the reaction rolls toward the product and the bond between A-B broke, and the bond between B-C was formed. The following plots will be obtained.
Jas213 (talk) 19:09, 28 May 2018 (BST)Very neat comparison.
Trajectories from r1 = rts, r2 = rts+δ,
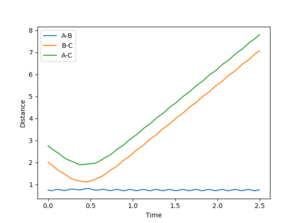
By setting r2 slightly higher than rts while keeping r1 = rts, atom C will move further away from the other two atoms to generate the reactant, A-B. The distance vs time plot and the momentum vs time plot obtained from dynamic calculation is exactly the same as the former part because the three H atoms are indistinguishable and the energy profiles for the reactants and products are the same in this reaction.
Inverse Plot
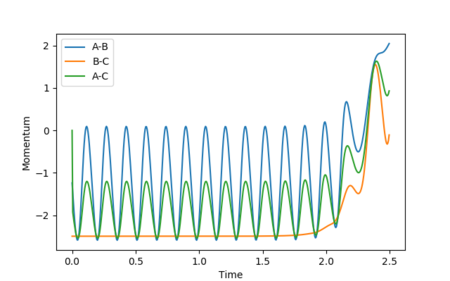
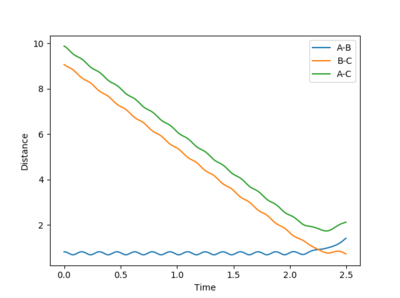
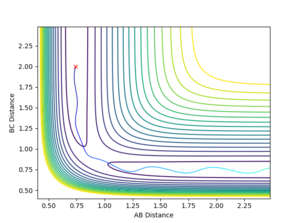
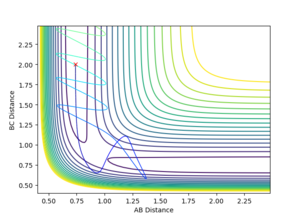
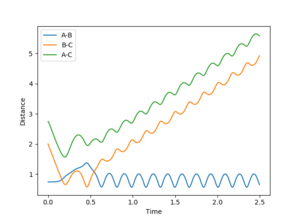
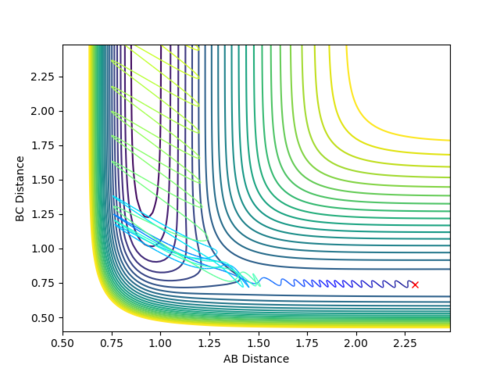
When the initial positions are setup to be the final positions of the trajectory from the internuclear distance vs time plot above, which are 0.810753 and 9.06604, and the momenta are setup to be the negative value of the average momenta at high t on the internuclear momentum vs time graph, which are -1.24756 and -2.4931, new calculations are generated. On the distance vs time plot, r1 and r2 line meet each other at around time 2.13. Therefore, it demonstrates that theses atoms have enough kinetic energy to overcome the activation barrier, so they pass the transition state to form the products.
Jas213 (talk) 19:10, 28 May 2018 (BST) Units units units.
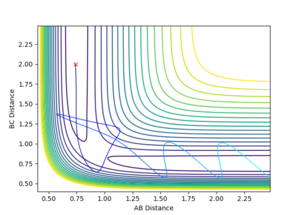
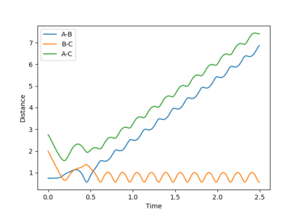
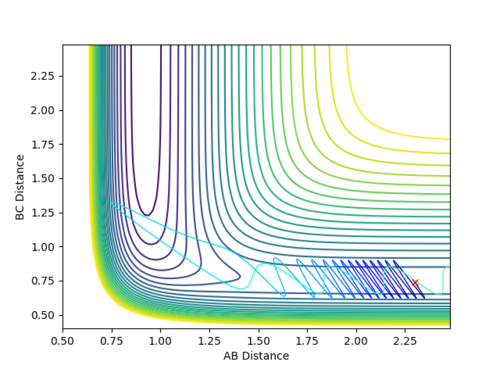
Reactive and Unreactive Trajectories
Jas213 (talk) 19:12, 28 May 2018 (BST) What about the wiggly line at the start of trajectory 3 compared to trajectory 1. Comments on vibrational/translational energy contribution and relating it back to the momenta applied are missing. An overall concluding comment on what you learned from the table would have been expected.
Transition State Theory
Assumption in transition state theory:
1. each intermediate is long-lived enough to reach a Boltzmann distribution of energies before continuing to the next step.
2. unless atoms or molecules collide with enough energy to form the transition structure, then the reaction does not occur.
3.the reaction system will pass over the lowest energy saddle point on the potential energy surface.
Second assumption stated that when the system possesses energy greater than or equal to the activation energy, the reaction will proceed to form the products from the reactants. However, the experiment 4 and 5 both have enough energy to overcome the activation energy, but experiment 4 is unreactive while 5 is reactive. This is because in experiment 4, the transition state theory overestimates the rate constant. This can be fixed by multiplying rate constants by a transmission coefficient.
Jas213 (talk) 19:13, 28 May 2018 (BST) Correct statements and discussion, but where are your references??
F-H-H system
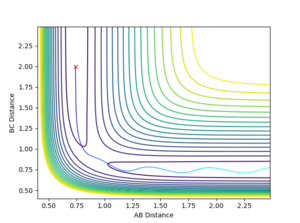
PES Inspection
The bond energy between H-H is 432 kcal/mol, and the bond energy between H-F is 565 kJ/mol. Therefore H-H bond is weaker and higher in energy. For F + H2 system, bond between H-H is broken, which absorbs energy, and bond between H-F is formed, which releases energy. Energy that has been released overwrites the energy that has been absorbed, so this is an exothermic reaction. Vice versa for the reverse reaction.
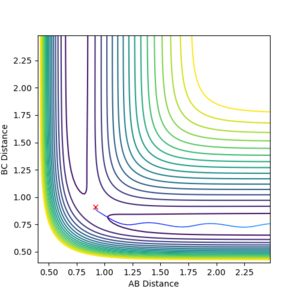
In this experiment, r1 is the distance between F and H atoms, and r2 is the distance between two H atoms. According to Hammond postulate, when the reaction is an exothermic reaction, the transition state is in close proximity to the reactant, which is H-H and F in this case, so r2 at the transition state should be shorter and more like H-H bond distance, and r1 is longer. The approximate positions of the transition state are r1 = 1.8115, and r2 = 0.7437.
The transition state for this reaction is at the minimum point of V( rHH ), but the maximum point of V( rFH). Therefore, if we set rFH (r1) larger than 1.8115, it will roll back towards the reactants, and if we set rFH to be slightly smaller than 1.8115, it will roll towards the products.
The activation energy for the F + H-H reaction is 0.262 kcal-1 , which is obtained from the energy vs time plot below. In this calculation, the H-F distance is set to be slightly loner than its distance at transition state. The least negative energy is -103.752 kcal-1 , and the most negative energy is -104.014 kcal-1 .
Jas213 (talk) 19:24, 28 May 2018 (BST) Where's the proof that you found a TS? Where is your distance vs. time plot or a MEP only showing a dot.

The activation energy for the H + F-H reaction is 30.247 kcal-1 , which is obtained from the energy vs time plot below. In this calculation, the H-F distance is set to be slightly shorter than its distance at transition state. The least negative energy is -103.751 kcal-1 , and the most negative energy is -133.998 kcal-1 .
Jas213 (talk) 19:15, 28 May 2018 (BST) Good discussion of how you obtained the TS, shows good understanding of the theory & experiment. However, units missing and Energy is not kcal-1 it is kcal/mol
Reaction dynamics
F + HH System
Polanyi's empirical rules state that transnational energy is more efficient to promote a reaction with early transition state.
Setting the distance between F and H to be 2.3, so they are apart and energy between them are translational energy. The H-H distance is set to be 0.74, which is the bond distance between normal H-H bond. Energy between them is dominated by vibrational energy.
If the pFH=-0.5, and the pHH=3, the reaction trajectory is unreactive although in this case the vibrational energy is very high.
If the pFH is increased to -0.8, and pHH is decreased to only 0.1, the reaction trajectory is reactive although in this case the vibrational energy is very low. This is because for the reaction with early transition state, the translational energy is the dominate factor to determine whether or not the reaction trajectory is reactive. And according to first law of thermodynamics, the energy is conserved. Therefore, the energy possessed by the reactants has been transformed to the kinetic energy for the leaving H atom and the vibrational energy for the new HF bond. On the graph, the vibrational energy of HF bond has larger amplitude than vibrational energy of HH bond, which means the translational energy between H and F has been transformed to the vibrational energy between HF.
H + HF System
Polanyi's empirical rules state that vibrational energy is more efficient to promote a reaction with late transition state.
Setting the rFH = 0.92, which is the normal F-H bond distance. And rHH = 2.3, so that two H atoms are far apart .
If pFH = -0.1, and pHH = 10, which is very high, H atom will hit the HF bond with very high kinetic energy so that it breaks the HF bond. However, the this H atom bounce off with also high kinetic energy, so the other H atom cannot react with it to form H-H bond. So the overall reaction is not reactive, and three separate atoms are hanging around.
Jas213 (talk) It looks like you are going in the wrong direction on your PES. -10?
If pFH = 10, and pHH = -0.1, the reaction is reactive. Because this reaction has a late transition state reaction, so the vibrational energy is the dominating factor to determine whether the reaction is active or not. The vibrational energy for the reactants is high enough, so that the reaction is active.
Jas213 (talk) 19:20, 28 May 2018 (BST) Good illustration of Polanyi's rules, shows good understanding of the topic; you should have given an overall conclusion.
References
1.Zhang Z, Zhou Y, Zhang DH, Czakó G, Bowman JM. Theoretical study of the validity of the polanyi rules for the late-barrier Cl + CHD3 reaction. J Phys Chem Lett. 2012;3(23):3416–9.
2.Masel, R. (1996). Principles of Adsorption and Reactions on Solid Surfaces. New York: Wiley
3.Pineda, J. R.; Schwartz, S. D. (2006). "Protein dynamics and catalysis: The problems of transition state theory and the subtlety of dynamic control". Phil. Trans. R. Soc. B. 361 (1472): 1433–1438. doi:10.1098/rstb.2006.1877. PMC 1647311 Freely accessible. PMID 16873129