MRD:jag115
Molecular Reaction Dynamics
EXERCISE 1: H + H2 System
Question A: What value does the total gradient of the potential energy surface have at a minimum and at a transition structure? Briefly explain how minima and transition structures can be distinguished using the curvature of the potential energy surface?
A transition state is a saddle point in the potential energy surface, it has a total gradient of zero in the potential energy surface. The saddle point is a maximum within the minimum energy path, if we move along the direction of the bond length decreasing we would observe the transition state as maxima however if we consider the transition state in terms of the potential energy we would observe a minima.
∂E/∂r = 0 for reaction coordinates, r, for both minima and for the transition state. For a transition state, ∂E2/dr2 = 0 for r of the reaction coordinate but >0 for all other values of r and for a minimum ∂E2/dr2 for all values of r.
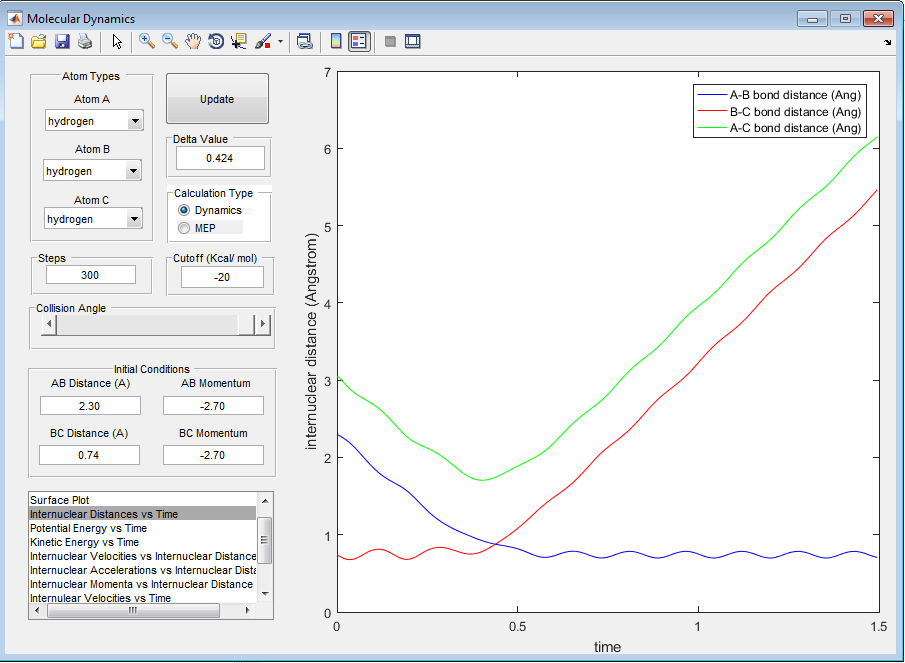
Question B: Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” screenshot for a relevant trajectory.
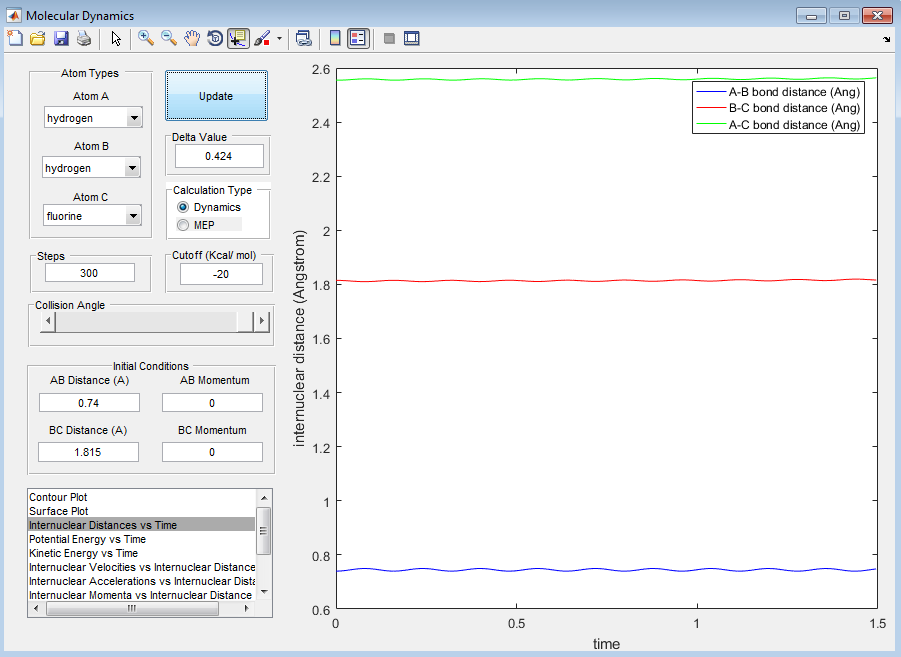
The value at which A-B bond distance equals B-C bond distance can be seen from the plot of 'Internuclear Distances vs Time', shown below.
From the above image it can be found that the distance at which A-B bond distance is equal to B-C bond distance is 0.907Å.
We can be sure we have found the transition state bond length as there is very little oscillation of the bond length which is what is expected in the transition state. I used trial and error to find the transition state bond length by looking at how the bond length oscillated.
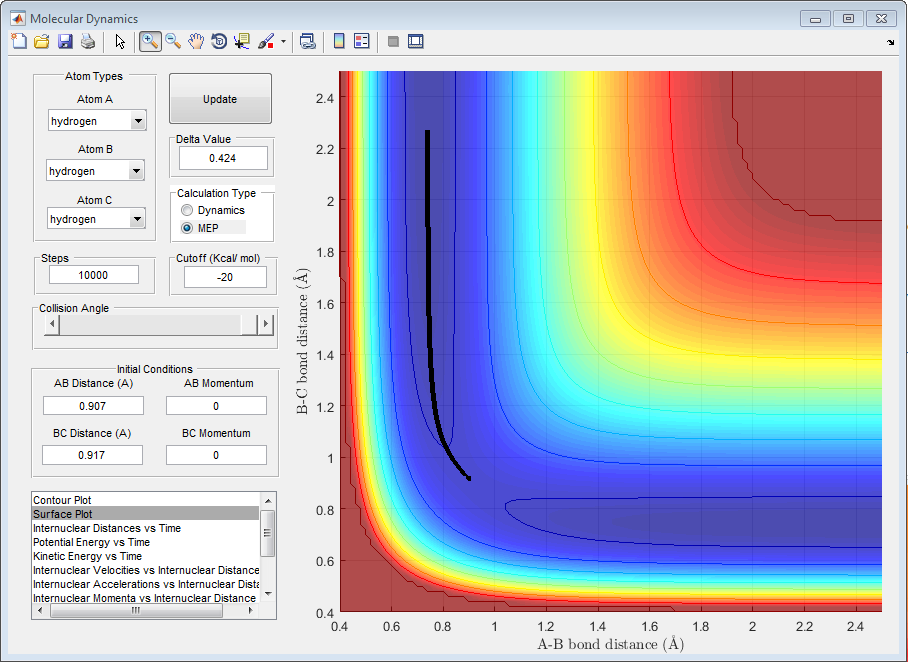
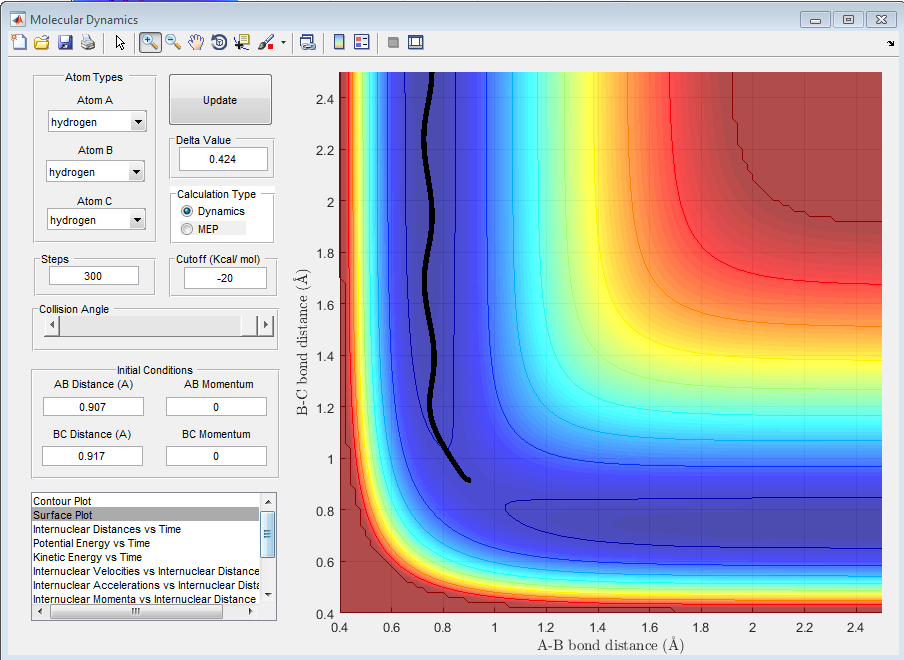
Question C: Comment on how the mep and the trajectory you just calculated differ.
r1 = 0.917 and r2 = 0.907
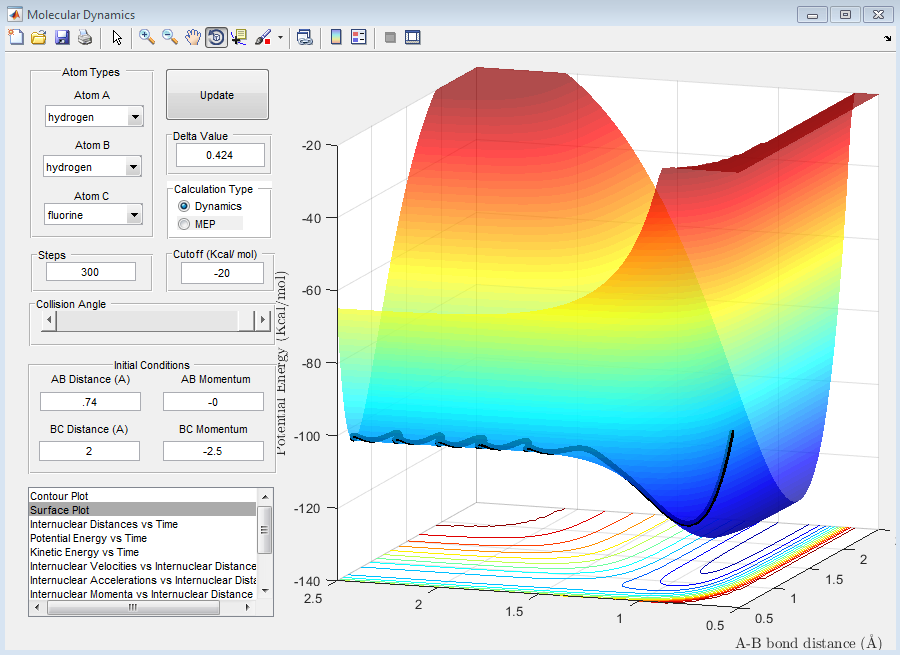
As can be seen from the above plots, the 'MEP' and 'Dynamic' calculations go through the minima of the valley. The crucial difference between the two plots can be seen from the lines, in the 'MEP' plot the line appears as a curve however in the 'Dynamic' calculation the lines is more waved-like, this is due to the calculations in the 'MEP' plot neglecting the vibrational energy of the molecules. In the 'Dynamic' plot, the changing vibrational energies of the molecules are apparent from the change in line shape.
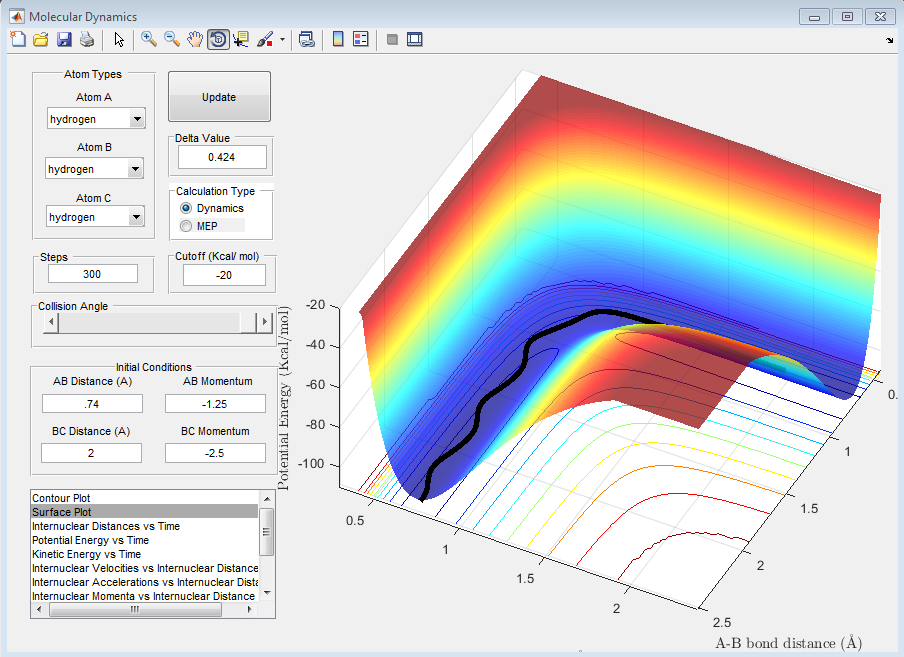
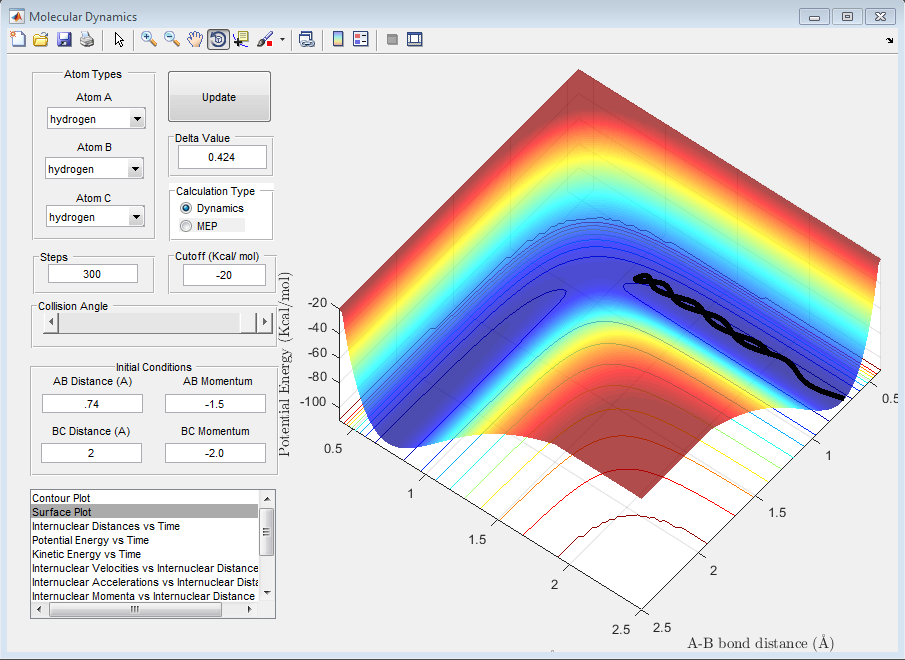
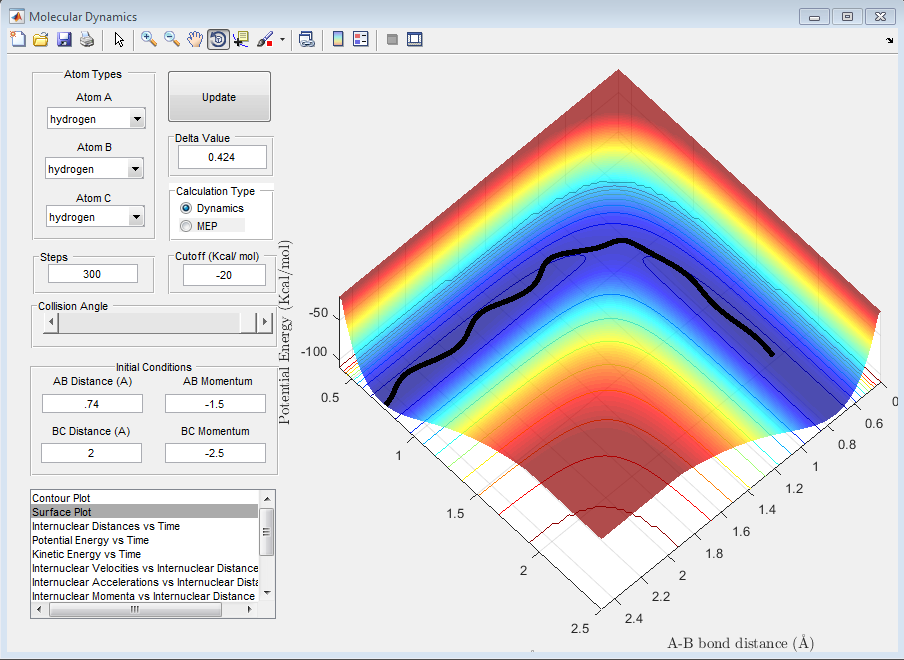
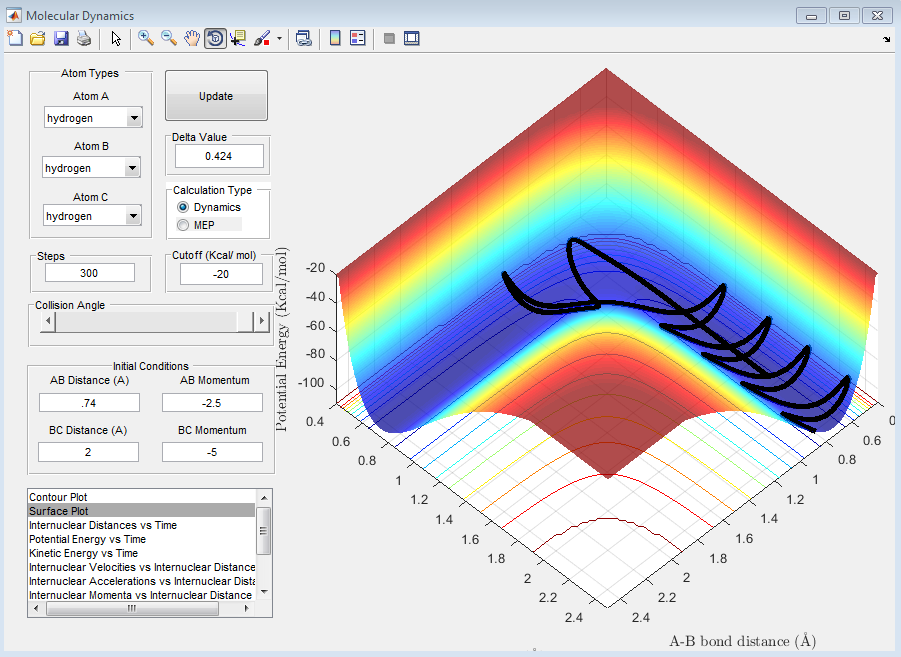
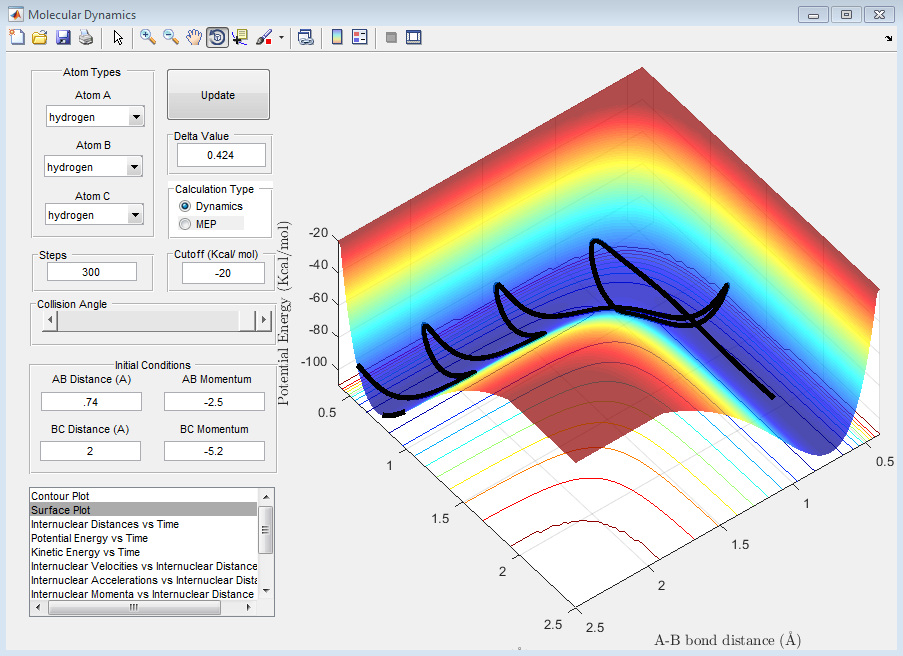
Question D: Complete the table by adding a column reporting if the trajectory is reactive or unreactive. For each set of initial conditions, provide a screenshot of the trajectory and a small description for what happens along the trajectory.
The values of r used were: r1 = 0.74 and r2 = 2.0
| Reaction Number | P1 | P2 | Reaction? |
|---|---|---|---|
| 1 | -1.25 | -2.5 | Yes |
| 2 | -1.5 | -2.0 | No |
| 3 | -1.5 | -2.5 | Yes |
| 4 | -2.5 | -5.0 | No |
| 5 | -2.5 | -5.2 | Yes |
The above trajectory, A, is reactive. It passes through the transition point an proceeds to the reactants.
The above trajectory, B, is unreactive. It passes through the transition point however bounces back to the reactants opposed to forming products.
The above trajectory, C, is similar to that of A and is reactive. It passes through the transition point an proceeds to the reactants.
The above trajectory, D, is unreactive. It passes through the transition point to products however it recrosses the transition point once more and reforms reactant.
The above trajectory, E, is reactive. It passes through the transition point however recrosses twice so is overall a reactive process.
Question E: State what are the main assumptions of Transition State Theory. Given the results you have obtained, how will Transition State Theory predictions for reaction rate values compare with experimental values?
In Transition State Theory, the reactants and products are assumed to be in quasi equilibrium i.e. where a process occurs slowly enough that the system remains in internal equilibrium. We further assume the Classical Transition State theory, in this it is assumed that the interactions between reactants can be described by classical mechanics and negates the quantum mechanical effects that can trigger reactions and hence the rates of reaction predicted using this model are lower than the empirically determined rates. We further assume that all molecules with energy greater than the required activation energy will react to form product however we know this is not the case as shown on the fourth figure in question D, the molecule has sufficient energy to react however the plot re-crosses the the transition state and reforms reactant.
EXERCISE 2: F - H - H System
Question A: Classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved?
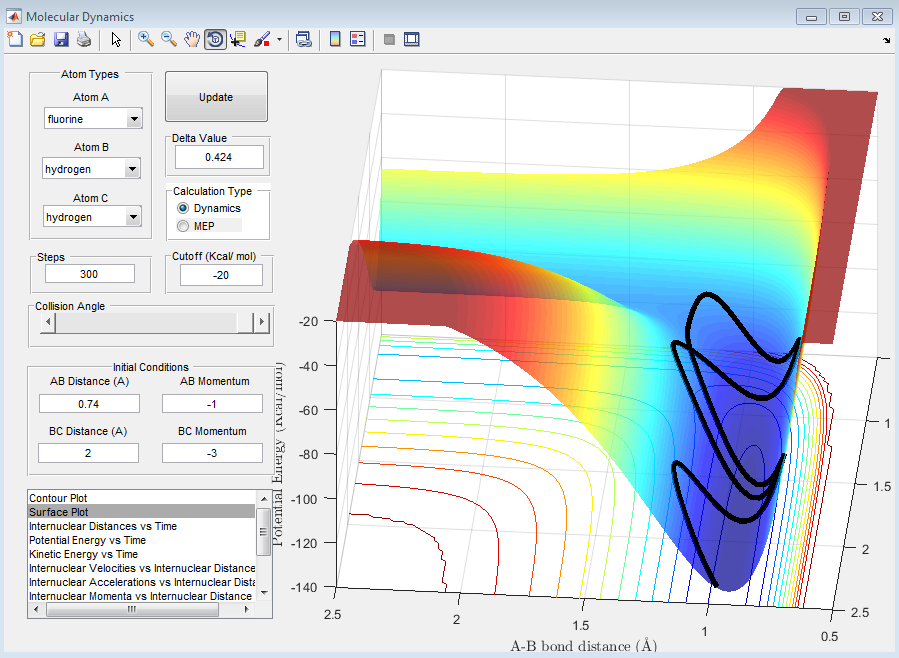
For the reaction of: 'F + H2 ---> FH + H' is represented by the figure below. The figure shows that the products are at a lower energy than the reactants so the reaction is exothermic.
For the reaction of: 'FH + H ---> H2' is represented by the figure below. The figure shows that the products are at a higher energy than the reactants so the reaction is endothermic.
| Bond | Enthalpy/(kJ/Mol) |
|---|---|
| H-H | 435 |
| H-F | 569 |
F + H2 ---> FH + H =-139kcal/mol (i.e. exothermic)
FH + H ---> F + H2 =139kcal/mol (i.e. endothermic)
Question B: Locate the approximate position of the transition state.
The approximate position of the transition state can be found as outlined in Exercise 1, Question B.
This shows the H-H-F distance.
Confirmation that the bond distance determined is that of the transition state as no oscillation can be seen.
Question C: Classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved?
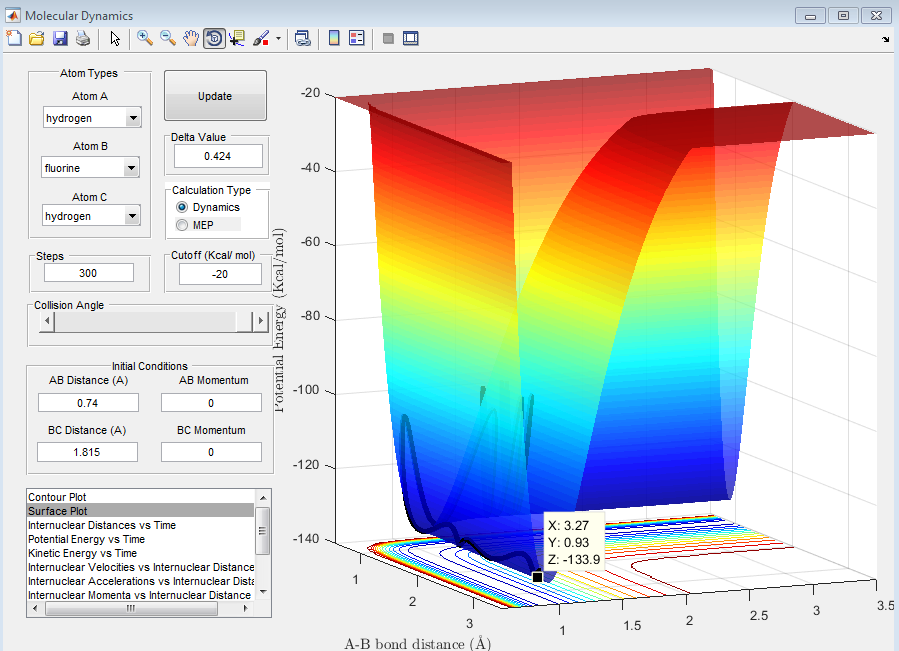
The activation energy is simply the difference in energy between the reactants and the transition state.
For H-F formation:
The above figure shows that the energy of the reactant, in this case H-H is -103.9 kcal/mol. This gives an activation energy of 0.2 kcal/mol.
The above figure shows that the energy of the reactant, in this case H-F is -133.9 kcal/mol. This gives an activation energy of 30.2 kcal/mol.
Question D: In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. How could this be confirmed experimentally?
The total energy of an isolated system such as the one we are investigating is overall conserved, potential energy is converted into kinetic and vibrational energy as the molecules collide. At the start of the reaction all of the energy is stored as potential energy within the molecule and as the molecules approach the transition state this potential energy is converted into kinetic and vibrational energy. If the reactants do not have sufficient energy when they collide, or collide in the wrong orientation, the reaction will not proceed. When two reactants collide successfully a transition state is formed in which vibrational and kinetic is converted to potential energy, the transition state has a maxima of potential energy. The transition state then separates to form the products and during this potential energy is converted into kinetic and vibrational energy once more.
By looking at the above surface plot for the reaction F + H2 ---> FH + H we can see that the the reactants have a greater potential energy than that of the products, hence the potential energy has been converted into vibrational and kinetic energy. The extent to which the energy has been converted into vibrational energy can be monitored by IR Spectroscopy.
Question E: Discuss how the distribution of energy between different modes (translation and vibration) affect the efficiency of the reaction, and how this is influenced by the position of the transition state.
Depending on if the reaction is endothermic or exothermic changes the structure of the transition state, in the sense that if it is an exothermic reaction the transition state will have a structure that more closely resembles the structure of the reactants, conversely if the reaction is endothermic the transition state will have a structure that more closely resembles that of the products. In an exothermic reaction, the one in which the transition state more closely resembles the structure of the reactants, for this reaction Polanyi's rules state a higher translational energy and lower vibrational energy make this type of reaction more likely. It should be noted however that if the translational energy is too high the reaction will proceed from reactants to products however it may also reverse, reforming the products. For the endothermic reaction, Polanyi's rules state that a higher vibrational energy and lower translational energy increase the likelihood of the reaction proceeding. Similarly to in the exothermic reaction, having too high of a vibrational energy could cause the products to reform reactants.
References
1. Dynamics of the Reaction of Methane with Chlorine Atom on an Accurate Potential Energy Surface. Gábor Czakó*, Joel M. Bowman. Science 21 Oct 2011: Vol. 334, Issue 6054, pp. 343-346
2. Computational Chemistry. Introduction to the Theory and Application of Molecular and Quantum Mechanics. Lewars, E.G. 2011, XVI, p. 664.
3. Atkins' Physical Chemistry. Atkins, P. and Paula, J. 10th edition. 2014.
4. http://www.kentchemistry.com/links/Kinetics/BondEnergy.htm Accessed on 11/05/2017 at 14:57