MRD:em2815
The H + H2 System
Note that in this computational investigation, A, B and C refer to HA, HB and HC, respectively, where HA is the incoming atom and HB and HC are part of the H2 molecule.
Locating the Transition State
Question 1
What value does the total gradient of the potential energy surface have at a minimum and at a transition structure? Briefly explain how minima and transition structures can be distinguished using the curvature of the potential energy surface.
The total gradient of both the potential energy surface at the minimum and the transition state is 0. These points can be distinguished by calculating the curvature of the surface using second order partial derivatives. The transition state is shown by the maximum on the minimum energy path which links the reactants and the products; this is also a saddle point. When calculating if both solutions are positive, it is a minimum, whereas if the solutions differ in sign, it is a saddle point, hence this is the transition state.
Question 2
Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” screenshot for a relevant trajectory.
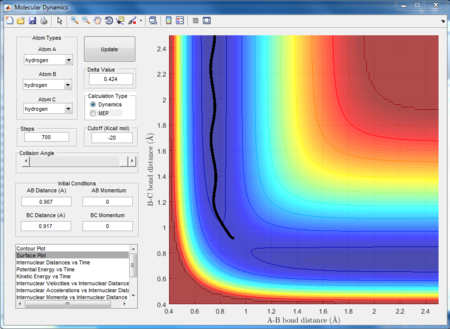
The best estimate of the transition state position is r1 (HB-HC) = 0.907 Å, r2 (HB-HA)= 0.907 Å. As can be seen by Figs 1a, 1b and 1c, at 0.907 Å the bond length stops oscillating and there is no divergence (it stays constant). At 0.8 Å it can be seen that the distance between the atoms diverges, showing that the reaction is proceeding to products. This means that it can not be the transition state because if it was, there would be no drive for the reaction to proceed towards reactants or products; the trajectory would oscillate on the ridge without diverging. Fig 1c shows that the bond lengths have not diverged thus the reaction is not proceeding towards reactants or products; the transition state has been reached at 0.907 Å.
 |
 |
|

The Reaction Path & Trajectories
Question 3
Comment on how the mep and the trajectory you just calculated differ.
Fig 2 and Fig 3 show the dynamic calculated trajectory and the mep, minimum energy path, respectively. In Fig 2, the line is oscillating, suggesting that the bond in the product molecule is vibrating. In Fig 3, the line is completely smooth, indicating that there is no vibration in the product molecule AB. This difference is due to the type of calculation taking place. In the dynamic calculation, the velocity of the atoms over the course of the reaction is taken into account therefore the molecule will be seen to be vibrating (hence the graph shows an oscillating curve). In the reaction path calculation, the velocity is reset to zero in each step therefore the molecules are measured when they are in infinitely slow motion. This means that they will not be seen to be vibrating, resulting in a smooth graph.
 |
 |
Question 4: Reactive & Unreactive Trajectories
Complete the table by adding a column reporting if the trajectory is reactive or unreactive. For each set of initial conditions, provide a screenshot of the trajectory and a small description for what happens along the trajectory.
| Trajectory | p1 | p2 | Reactivity |
|---|---|---|---|
| 1 | -1.25 | -2.5 | Reactive |
| 2 | -1.5 | -2.0 | Unreactive |
| 3 | -1.5 | -2.5 | Reactive |
| 4 | -2.5 | -5.0 | Unreactive |
| 5 | -2.5 | -5.2 | Reactive |
 |
 |
 |
 |
 |
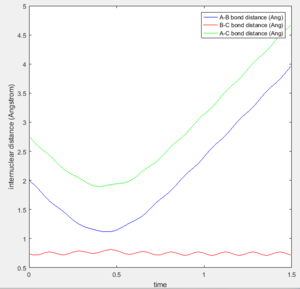
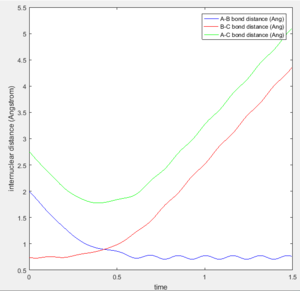
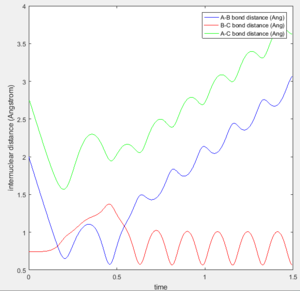
Figures 4a-4e show the trajectories with differing initial momenta. It can be seen that trajectories 1, 3 and 5 are reactive, whereas trajectories 2 and 4 are unreactive. This can be deduced from the Internuclear Distance vs Time plot. For a successful reaction, it is expected that as the A atom approaches B, the distances AB and AC will decrease until the transition state is reached. Note that at this point, the distance BC will stay constant. As the reaction proceeds and the A-B bond forms, the distance AB will oscillate due to vibrations alongside an increase in the distances BC and AC as the C atom moves away. This is exactly what is depicted in Figures 4a, 4c and 4e, indicating that the product has been formed and the trajectories are reactive.
Figures 4b shows the data obtained for trajectory 2. This figure shows that the AB and AC distances decrease as the A atom approaches the B atom during the start of the reaction. After this, the AB and AC distances start to increase, implying that A is moving away from B rather than forming a bond. It can be seen that the transition state is not formed in this case and the B-C bond consistently vibrates throughout the reaction, hence the trajectory is unreactive.
Figure 4d shows the data obtained for trajectory 4. Like Fig 4b, it shows that the AB and AC distances decrease as A approaches B but in this case the transition state is reached. During the start of the reaction, the BC bond lengthens as expected, however after the transition state has been reached, it shortens again before oscillating. This shows that the B-C bond has not been broken and it stays intact, hence the product AB has not formed and the reaction has proceeded back to the reactants. This process is known as barrier recrossing.
Question 5: Transition State Theory
State what are the main assumptions of Transition State Theory. Given the results you have obtained, how will Transition State Theory predictions for reaction rate values compare with experimental values?
Transition state theory assumptions:
- Assumption 1: The supermolecule, H3, will proceed to products if it has crossed the critical dividing surface from the reactant side - this is the boundary which passes through the saddle point of the potential energy surface. [1]
- Assumption 2: The Boltzmann distribution of energy of reactant molecules stays constant during the reaction. [1]
- Assumption 3: Molecules of H3 which cross the critical surface from the reactant side will have a Boltzmann distribution of energy which relates to the temperature of the system. [1]
Due to assumption 1, it can be said that the Transition State Theory will predict a higher reaction rate than those obtained experimentally. The assumption predicts that if the transition state is crossed from the reactant side, the reaction will definitely proceed to products. Trajectory 4 shows that this is not the case, since the transition state has been crossed but the reaction has proceeded back to reactants rather than forming the products. This confirms that experimental investigation leads to a lower reaction rate.
The F-H-H System
Reaction Energetics
Question 6
Classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved?

When changing the atoms to F, H and H, the surface plot generated (Fig 5) shows that the potential energies of H-H + F and H-F + H are -103.9 kcal/mol (-434.7 kJ/mol) and -133.9 kcal/mol (-560.2 kJ/mol), respectively. This shows that the forward reaction H-H + F → H-F + H has a ΔE of -125.5 kJ/mol and the backward reaction H-F + H → H-H + F has a ΔE of +125.5 kJ/mol; hence the forward reaction is exothermic and the backward reaction is endothermic. This is reliable since it is consistent with the literature value from a study by C.F. Bender et al. which states that the magnitude of the energy gap is 143.9 kJ/mol. [2]
This makes sense when considering the bond strength of the species involved. The H-F bond is extremely strong so when it is made during the forward reaction, more energy will be given out than is taken in when breaking the H-H bond and thus the reaction is exothermic. During the backward reaction, more energy will be taken in to break the strong H-F bond than will be given out making the H-H bond, hence the reaction is endothermic.
Question 7
Locate the approximate position of the transition state.
Through the process of trial and error, the transition state was located at r1(H-H) = 0.745 Å, r2(H-F) = 1.810 Å. This is the position at which the distances are constant without oscillation (see Fig 6), therefore the transition state must be located here (for the reasons explained above).

Question 8
Report the activation energy for both reactions.
Calculation of activation energies:
The energy of the transition state is -103.3 kcal/mol = -432.2 kJ/mol (see Fig 7)
The energy of the reactants (H-H + F) is -103.9 kcal/mol = -434.7 kJ/mol
The energy of the products (H-F + H) is -133.9 kcal/mol = -560.2 kJ/mol
Therefore the activation energy of the forward reaction H-H + F → H-F + H is 2.5 kJ/mol and the activation energy of the backward reaction H-F + H → H-H + F is 128.0 kJ/mol.

Reaction Dynamics
Question 9: Conservation of Energy
In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. How could this be confirmed experimentally?
After identifying a set of conditions which results in a reactive trajectory, it can be seen that the product bond momentum (H-F) oscillates (see Fig 8). This shows that the bond is vibrating, indicating that energy is conserved through the release of vibrational energy. The release of energy can be investigated experimentally by using calorimetry.

Question 10: Polanyi's Rules
Discuss how the distribution of energy between different modes (translation and vibration) affect the efficiency of the reaction, and how this is influenced by the position of the transition state.
Polayni's rules:
Polanyi's rules state that vibrational energy is more efficient than translational energy in promoting a late transition state (one resembling the products), whereas for early transition states (those resembling the reactants), translational energy is more efficient than vibrational energy. [3]
This indicates that for the forward reaction H-H + F → H-F + H, since the transition state is early (see Fig 7) , an increase in translational energy will increase the efficiency of the reaction. Conversely, for the backward reaction H-F + H → H-H + F, since the transition state is late, an increase in vibrational energy will be more efficient.
Nf710 (talk) 17:51, 2 June 2017 (BST) You havent proved this and therefor you cant just state it.
Nf710 (talk) 17:52, 2 June 2017 (BST) Nice concise report but you are missing a few things
References
<references> [1]
</references
- ↑ 1.0 1.1 1.2 1.3 Levine, Ira N. Physical Chemistry. 6th Ed., International ed. Boston, [Mass.] ; London: McGraw-Hill Higher Education, 2009.
- ↑ 2.0 2.1 Bender CF, O'Neil SV, Pearson PK, Schaefer HF. Potential energy surface including electron correlation for F+ H2→ FH+ H: refined linear surface. Science. 1972 Jun 30;176(4042):1412-4
- ↑ 3.0 3.1 Zhang Z, Zhou Y, Zhang DH, Czakó G, Bowman JM. Theoretical study of the validity of the Polanyi rules for the late-barrier Cl+ CHD3 reaction. The journal of physical chemistry letters. 2012 Nov 9;3(23):3416-9.
