MRD:ck2917
MRD WIKI REPORT
Molecular Reaction Dynamics in Triatomic Systems
EXERCISE 1: H + H2 system
Question 1:
On a potential energy surface diagram, how is the transition state mathematically defined? How can the transition state be identified, and how can it be distinguished from a local minimum of the potential energy surface?
The mathematical definition of a transition state on a potential energy surface is defined as a maximum saddle point (or point of inflection) or the saddle point where the minimum energy pathway is at a maximum. A condtion for it to occur is when rab = rbc which is seen on the internuclear distance vs time graph as it is a function of both. The following conditions must be satisfied.
1)Vx=0 and Vy=0
2)VxxVyy-(Vxy)2<0
let: x=rab and y=rbc.
It can be distinguished by a local minimum as they both satisfy the first conditon however a local min will not satisfy the second conditon.

Question 2:
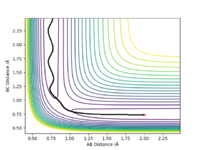
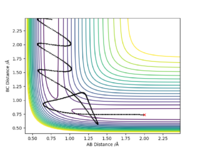
Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” plot for a relevant trajectory.
The closest value to zero energy change in order to locate the transition state geometry was estimated to rts = 0.9079 Å. This was done by trial and error analysis by observing the Internuclear Distances vs Time plot by altering the internuclear distances by small increments while keeping them equal to eachother and keeping momentum of the two at zero. From the graph below it can be see that no vibrations are present as both rab and rbc are presented by straight lines. Hence allowing for the deduction that as the internuclear distances remain unchanged with time, the only type of energy present is potenial energy (no KE).

Ng611 (talk) 13:45, 7 June 2019 (BST) Good!
Question 3:
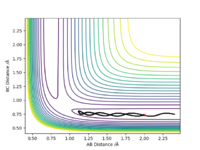
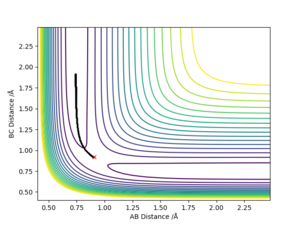
Comment on how the MEP and the trajectory you just calculated differ.
An MEP trajectory is smoother than in the dynamic trajectory upon comparing the two contour plots. Since the MEP is a trajectory ignoring the momenta of atoms that would be gained by deviations from the TS and also their velocities, KE = 0. Therefore, as atoms would 'roll down' with deviations from the TS, their momentum gained is negligible, and no kinetic energy present to cause the atom to travel, it does not oscillate and hence the smooth trajectory presented. By using the dynamic approach, a more realistic motion is illustrated for atomic motion since their is a non-zero momentum present. This results in oscillations / vibrations of the atom which is present in the figure below. Also note that in the MEP approach the molecule travels a smaller distance in the potential energy surface as momentum is lost.
Ng611 (talk) 15:45, 7 June 2019 (BST) Not deviations from the TS, but deviations from the minimum energy path (the reaction channel). Otherwise, this is a good answer. Also, remember that in the steepest descent approach of the MEP, momentum isn't lost (as that would imply that there's momentum in the system that's being dissipated), but totally neglected.

Reactive and unreactive trajectories
Complete the table above by adding the total energy, whether the trajectory is reactive or unreactive, and provide a plot of the trajectory and a small description for what happens along the trajectory. What can you conclude from the table?
It can be concluded that for a successful reaction to occur, the combined momenta p1 and p2 must match. As a result the variations of energies that either the single atom or the diatomic possess, will affect the reactivity greatly. A notable point is that a great amount of energy does not necessarily mean that a reaction will go to completion as illustrated by 4.
Question 4:
State what are the main assumptions of Transition State Theory. Given the results you have obtained, how will Transition State Theory predictions for reaction rate values compare with experimental values?
Transition State Theory (TST) consists of 3 main assumptions for its derivation.
- Reactants are in constant equilibrium with the transition state structure.
- The energy of the particles follow a Boltzmann distribution.
- Once reactants become the transition state, the transition state structure does not collapse back to the reactants.
The 3rd assumption is one we can associate to the calculation conducted above. These assumptions explain the rate of reaction but predicted rate is not in agreement with the above. TST prompts a classical view of the situtation where as when considering quantum mechanical effects theory and experiment are in disagreement. As illustrated by reaction 4, the reactants can overcome the TS to form a products but also rolled back on themselves to dissociate back into reactans, something that TST does not predict. This is a manifestation of quantum mechanical tunneling.
Ng611 (talk) 15:49, 7 June 2019 (BST) NO! Tunneling is a completely different effect that is totally ignored in these simulations. TS recrossing and tunneling are separate effects that affect the system in opposite directions. Tunneling increases the true rate relative to the TST prediction, whilst recrossing slows the true rate relative to TST.
EXERCISE 2: F - H - H system
Question 5:
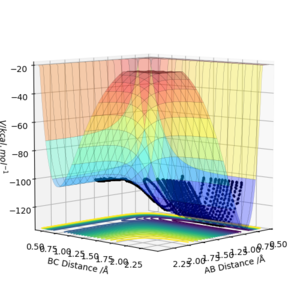
By inspecting the potential energy surfaces, classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved?
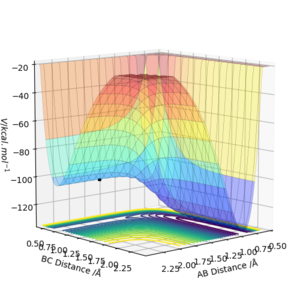
By inspection of the potenial energy surface below it can be deduced that the potential well decreases in energy as reacion progresses showing that the forward reaction is exothermic ( F + H2 --> H + HF ) and that the backward reaction is endothermic. This is because the products are at a lower energy than reactants, suggesting the stabily and strength of the newly formed H-F bond compared to the initial H-H bond. An exothermic reaction releases energy to the environment.
Question 6:
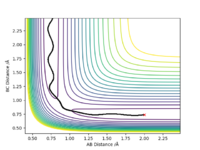
Locate the approximate position of the transition state.
By conducting trial and error analysis the most accurate approximation for the position of the TS was found to be at rAB = 1.812565 Å and rBC = 0.744841 Å. Initially a starting point was located after inspecting the contour plot to give a rough estimate of the TS position using the MEP. Then, the interatomic distances were altered until the shortest MEP trajectory was found confirming that the 2 r's (coordinates) on the plot were elucidating the position of the TS. The animation technique further confirmed the position as all three atoms were at rest.
Question 7:
Report the activation energy for both reactions.
In order to be able to calculate activation energies, the energy position of the reactants and products had to be calculated. By measuring their differences in energy compared to the energy of the TS this could be done. By changing interatomic distances to rAB = 1.9 Å and rBC = 0.7 Å as to ensure that atoms aren't sitting on the TS and roll back to the reactants side, reactants energy could be deduced from an MEP energy vs time graph. For the reverse reaction's activation energy to be calculated interatomic distance for rAB was set to 1.8 Å and pab was set to -0.35 so that the reaction could proceed downhill towards the products side. Again an energy vs time graph confirmed the relative energy positions.
Transition State energy = -103.752 kcal/mol Reactants energy = -104.013 kcal/mol Products energy = -133.773 kcal/mol
Therefore:
-
1.
- F + H2 ----> H + HF
- Εa = (-103.752) - (- 104.013) = 0.261 kcal/mol
2. - H + HF ----> F + H2
- Εa = (-103.752) - (- 133.773) = 30.021 kcal/mol
Ng611 (talk) 15:49, 7 June 2019 (BST) Good!


Question 8:
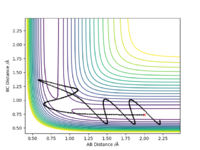
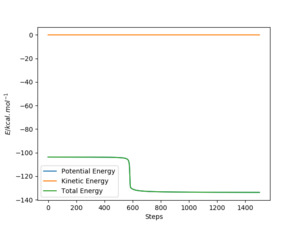
In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. Explain how this could be confirmed experimentally.
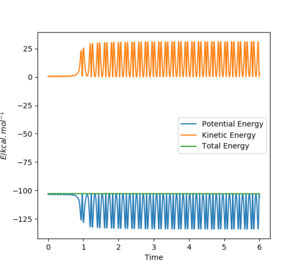
Firstly momentum was changed so that a succesful reaction was created to be able to study it. As we determined previously, the overall forward reaction is exothermic, and overall energy is being conserved. The excess energy that the products possess is converted into a large amount of vibrational energy within the HF molecule and can be seen distinctively from the PES and the contour plot depicted below. From an energy vs time plot, it is shown that total energy is constant and that it is always the sum of the combinations in potential and kinetic energies within the system. The momentum vs time plot illustrates the large amount of momentum present in the product after the reaction proceded further indicating the vast amount of vibrational energy present. This extra vibrational energy is the source of heat which is released upon exothermic reactions hence a way to confirm this reaction completion could be calorimetry in order to quantify the amount of energy liberated.
Ng611 (talk) 15:51, 7 June 2019 (BST) Calorimetry doesn't distinguish between vibrational and translational KEs. Can you think of a technique that does?


Question 9:
Discuss how the distribution of energy between different modes (translation and vibration) affect the efficiency of the reaction, and how this is influenced by the position of the transition state.
The Polanyi rules, state that vibrational energy is more efficient in promoting a late-barrier reaction than translational energy. (Z. Zhang ,Y. Zhou, D. H. Zhang, G. Czakó, J. M. Bowman,J. Phys. Chem. Lett.2012,3,23,pp3416-3419)
By using the Hammond's postulate, a late barrier transition is thought to be endothermic. This means that the vibrational energy which results from the momentum possessed by the diatomic; in this case HF, contributes more and is more significant in the reaction between HF and F. Conversely, when considering the exothermic process; H2 and F, the translational motion of the Fluorine atom is more significant. Polanyi rules were confirmed though the experiment by testing a range of values in order for a reaction to be completed successfully. When the momentum of the H molecule was set high (Translational energy) for the endothermic process reaction was not succesful. Upon swapping the momenta (high for HF and low for H), the reaction proceeded which is in line with what the rules suggest. It can also be deduced that activation energy isn't the only factor contributing to the reaction but it is the energy allocated to the correct type of mode (vibration vs translation).
Ng611 (talk) 15:53, 7 June 2019 (BST) Where is your trajectory for HF+H?