MRD:cid-01554120
Question 1
Mathematical definition of transition state
When modelling a potential energy surface of a chemical reaction, the transition state is a point on the minimum energy reaction pathway with maximum potential energy. Firstly, it is in the minimum energy pathway,so it is a minimum perpendicular to the reaction pathway. then, maximum potential energy appears at this point, means it is maximum alone the reaction pathway[1]. This sort of point are mathematically defined as a saddle point of a surface.
Identification of transition state
As the transition state can be represent by a saddle point, it is possible to apply mathematical methods[2] of identifying stationary points to determine whether a stationary point represent the stationary point or not.
| type of stationary points | identification methond |
|---|---|
| local maximum | , |
| local minimum | , |
| saddle point |
For a potential surface V(r1, r2). In order to identify if a point with certain reaction coordinates represent the transition state or not, we can substitute the reaction coordinates into the function . If the result is negative, it is the saddle point if the potential surface, and can represent the transition state.
Good description on distinguishing from the local minimum but your titled “mathematical definition of the transition state" (TS) is not very mathematical, you could mention that the TS is when the partial derivative of V(r1 and r2) is zero Sf3014 (talk) 22:47, 31 May 2020 (BST)
Question 2
Transition state position estimation
For the reaction of a hydrogen atom collides with a hydrogen molecule at 180 degree to form a new hydrogen molecule and release an other hydrogen atom, consider there are only two degrees of freedom involved in the function of potential surface V(r1, r2), where r1 is the distance between the free atom and one end of the molecule, and r2 is the distance between the two H atoms originally in the hydrogen molecule. The system is symmetric, so r1 and r2 are equal at the transition state. Secondly, for a stationary point, we have . The first derivative of potential is force. Therefore, in order to find the best estimation of the transition state. We need to run estimations with different r1 and r2, until we find the force alone the direction of r1 and r2 are both 0. The modelling starts from r1=r2=230pm(the initial distance between the free H atom and hydrogen molecule), and the best estimation is r1=r2=90.775pm.
Good but show evidence from your simulations to support your description Sf3014 (talk) 22:47, 31 May 2020 (BST)
Question 3
Reaction trajectories calculation
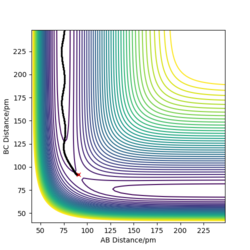 |
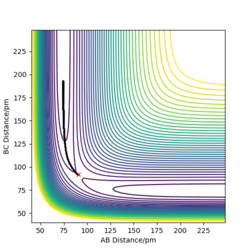
|
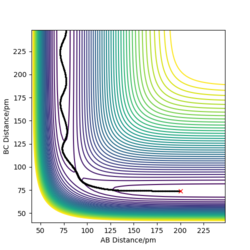
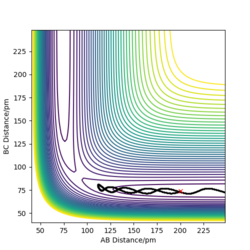
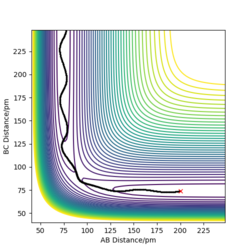
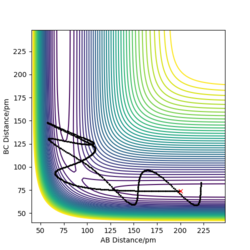
The picture on the left hand side shows the reaction trajectory calculated under dynamic type, it includes the vibrational level of the molecules after they enter the product side. the picture on the right hand side is the reaction trajectory under mep type calculation, and the vibrational levels are not included within this calculation.
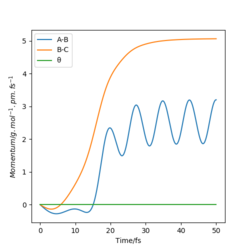 |
|
The picture on the left is the momenta-time graph under dynamic type calculation, the momenta of r1 and r2 are included in this calculation. The picture on the right is momenta-time graph obtained under mep type calculation, which ignored the momenta of r1 and r2
Very good comparison but more clarity can be gained from labeling your figures and referring to them by their labels Sf3014 (talk) 22:47, 31 May 2020 (BST)
Question 4
in this section A represent the original free H atom, B is the H atom in hydrogen molecule that collide with A, and C the H atom leaving the molecule after collision.
According to the table, momenta of molecules become more negative from the top to the bottom. It will be not appropriate to conclude a relation between reactivities and momenta(which represent the initial speeds of molecules). However, the total energies become less negative with more negative momenta. So we can conclude that energy can not solely determine the reactivity of an reaction.
Good table layout but in some cases the description could be more detailed, stating that the reaction is successful is not enough. Also, Your conclusion is unclear, are you talking about the combined momenta? Sf3014 (talk) 22:47, 31 May 2020 (BST)
Reference
1. Dill KA, Bromberg S. Chapter 19, Chemical Kinetics & Transition States. In:Molecular Driving Force: Statistical Thermodynamics in Biology, Chemistry, Physics and Nanoscience.2nd ed. United States of America: Garland Science, Taylor & Francis Group, LLC; 2010. p.364-365.
2. Steiner E. Chapter 9, Functions of Several Variables, In: The Chemistry Maths Book. 2nd ed. United States: Oxford University Press; 2008. p.253-255.