MRD:chengwang01492455
H + H2 system
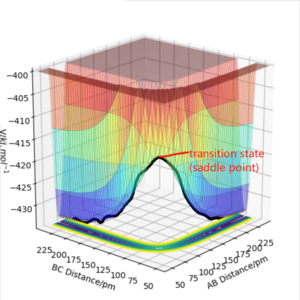
On a potential energy surface diagram, how is the transition state mathematically defined? How can the transition state be identified, and how can it be distinguished from a local minimum of the potential energy surface?
-- It is the saddle point in potential energy surface,at this point the slope in all orthogonal directions are all zero, ∂V(ri)/∂ri=0. It is the maximum on the minimum energy path linking reactants and the products(Figure 1). The point starts a trajectory exactly at the transition state, with no initial momentum, it will remain there forever. In addition, according to the Hammond's postulate, if reaction is exothermic, the transition state will resemble the reactants, while if the reaction is endothermic, it will resemble the products.
-- The difference between local minimum and transition state :
The transition state is neither minimum or maximum.For local minima,its second derivative is always >0, while at transition state, because it is saddle point, the second partial derivative with respective to one axis is negative, while to another axis will be positive, which means at the transition state, it has local minima in one direction but local maxima in the other direction(orthogonal to previous one).
Hessian at the point can also be used to distinguish transition state from the local minimum. At transition state, It will have negative eigenvalues of hessian along one direction (In Figure, red line), while positive eigenvalues of hessian along the direction orthogonal to the previous one(In Figure, blue line). At local minima, hessian will always be positive defined with all eigenvalues are positive (positive curvature).
Nice and (for my taste) complete description, looks like you understood it. Fdp18 (talk) 09:35, 9 May 2020 (BST)
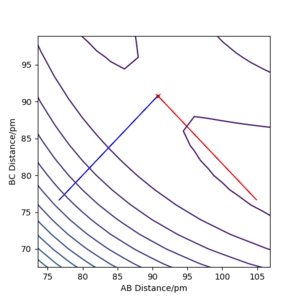
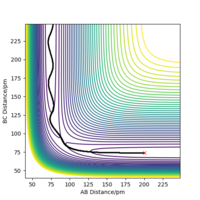
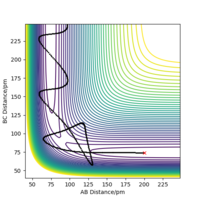
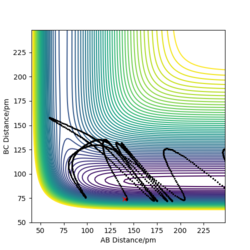
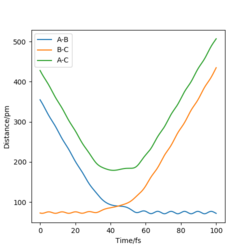
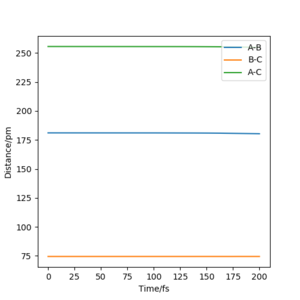
Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” plot for a relevant trajectory.
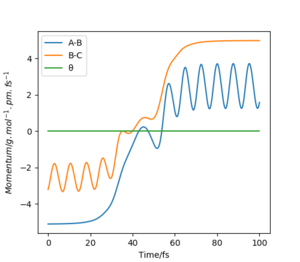
H+ Hr2 surface is symmetric, According to Hammond's postulate transition state will be r1= r2, and p1 = p2 = 0.0 g.mol-1.pm.fs-1.
Best estimate of the transition state position (rts): 90.775 pm
At this symmetric transition state, as gradient of potential energy surfaces equals to zero, its initial forces are also 0 Kg.mol-1.pm-1.
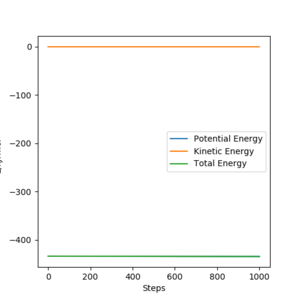
The eigenvalues of hessian show one positive and one negative (Figure 2).
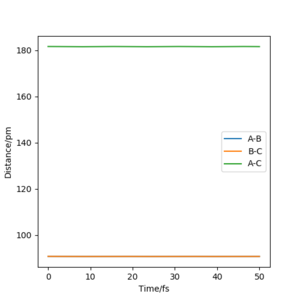
(Figure 3) illustrated, the r1 and r2 will stay constant at certain value, meaning the atoms are no longer oscillating at this transition state.
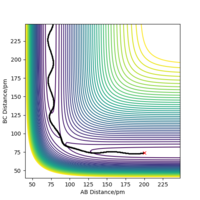
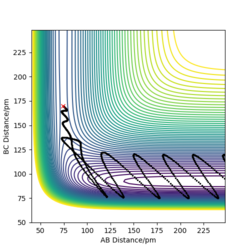
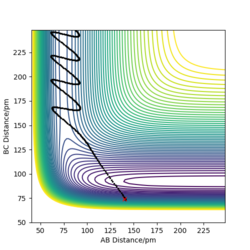
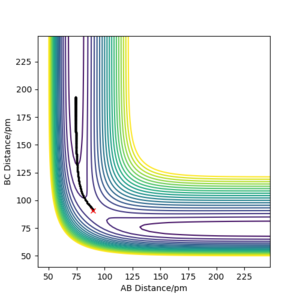
Comment on how the mep and the trajectory you just calculated differ.
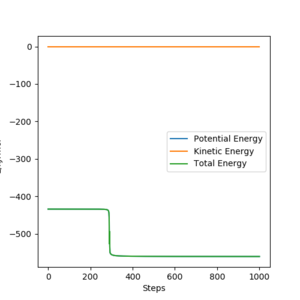
The reaction path mep is set with system is slightly displaced from the transition states (r1 = rts+1 pm=91.775 pm, r2 = rts=90.775 pm and the momenta p1 = p2 = 0 g.mol-1.pm.fs-1)
Difference:
Wavy line was seen in dynamics trajectory where there is smooth and shorter path shown in mep.
No vibrational energy for mep--not oscillating for mep.
Velocities are always reset to zero in each time step, so kinetic energy is zero in mep -- path is shorter than dynamic trajectory.
Total energy in system is always constant in dynamics simulation, while total energy in system is decreased in mep (kinetic energy is always zero, while potential energy is decreased.)
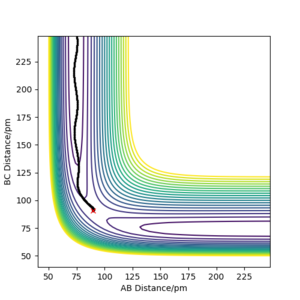
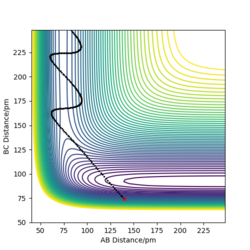
r1 = rts+1 pm=91.775 pm, r2 = rts=90.775 pm ,p1=p2=0
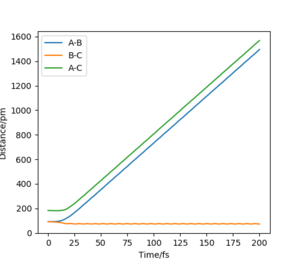
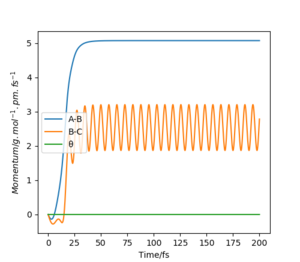
r1 = rts=90.775 pm, r2 = rts+1=91.775 pm. p1 = p2 = 0.0 g.mol-1.pm.fs-1
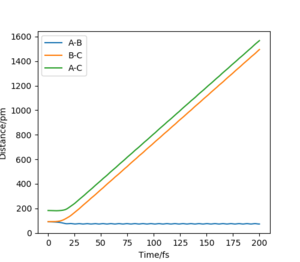
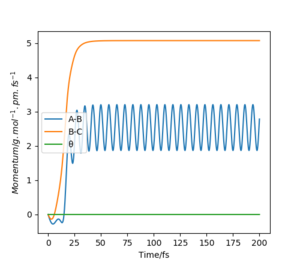
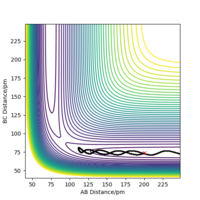
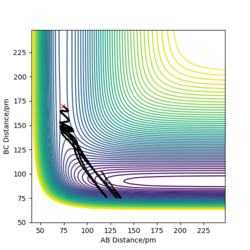
When swapping the initial condition of r1, r2. From the “Internuclear Distances vs Time”and “Momenta vs Time”, it can be seen that the curve of r1, r2 swap the position,but remaining the same trend.
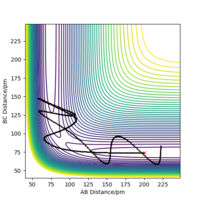
Set the initial position to final value of above trajectory(reverse the sign of final momentum)
r1=352.59 pm,r2=74.04 pm, p1=-5.07g.mol-1.pm.fs-1 p2=-3.20g.mol-1.pm.fs-1
As the new plot shows the trajectory will initially return pack to the r1 =90.775 pm,r2 = 91.775 pm, no reaction takes place, the system will then return back to its initial position.
well done, no problems here apart from 1-2 typos (Though I probably have more in my comments). Fdp18 (talk) 09:40, 9 May 2020 (BST)
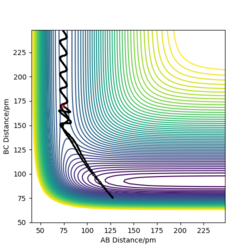
Complete the table above by adding the total energy, whether the trajectory is reactive or unreactive, and provide a plot of the trajectory and a small description for what happens along the trajectory. What can you conclude from the table?
Conclude from the table From the table, smaller p1/p2 ratio will result in higher possibility of reactive trajectory. Higher value of momentum will result in more complicated trajectory, its energy will also more likely towards positive value.
Given the results you have obtained, how will Transition State Theory predictions for reaction rate values compare with experimental values?
The main assumption of transition state theory states:(reference from: Laidler, Keith J, Chemical kinetics,pp. 88-98)
<1> molecules systems in direction of products cannot return back and form reactants molecule again.
<2> The energy distribution among the reactants molecule is same as Boltzmann distribution.
<3> It is permissible to separate the motion of system over the col from the other other motions associated with the activated complex. I can't understand this sentence... 'col' as in collective variable? Fdp18 (talk) 09:45, 9 May 2020 (BST)
<4> A chemical reaction can be satisfactorily treated in terms of classical motions over the barrier, quantum effects being ignored.
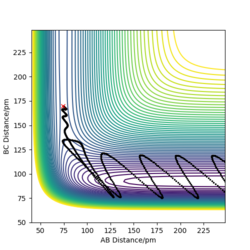
Assumption <1> will overestimate the rate. In TST, it count anythings across the activation barrier,but in real reaction, there is presence of recrossing, the products could reform the reactants, no reaction is happened, e.g. surface plot D, it initially forming the new molecule HAHB(reactants have crossed transition barrier), but HB will go back to HC, forming reactants HCHB agian.
Assumption <4> will underestimate the rate. There is presence of tunneling effect in real reaction, especially for light molecules, they don't need to go over high activation energy.
Generally, recrossing is more significant than tunneling effect, TST overestimate the rate.
Well done, both tunneling and recrossing are considered, as well as their relative significance. Fdp18 (talk) 09:45, 9 May 2020 (BST)
F - H - H system
By inspecting the potential energy surfaces, classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved?
Locate the approximate position of the transition state.
F + H2: when the initial conditions is rFH= 130 pm, rHH=74 pm, pFH=0 g.mol-1, pHH=-0.5g.mol-1, it can be seen from the surface plot that system will undergo reactive trajectory,reactants F + H2 are higher in energy than the products H + HF, the total energy is negative at -440.229kJ.mol -1. it indicates the F + H2 reaction is exothermic.
Therefore reaction H + HF is endothermic, reactants H + HF are lower in energy than the products F + H2.
It also states that the bond strength of H-F is stronger than H-H, so forming H-F bond release large amount of the energy,resulting in exothermic reaction.
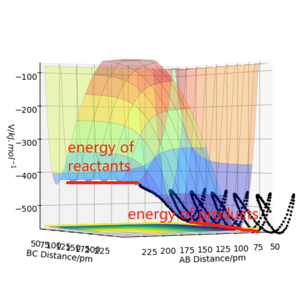
According to Hammond's postulate, the transition state will more likely to resemble the reactants in F + H2 reaction, because it's exothermic. rHHwill be shorter in length.
Approximate position of transition state is rFH=181.2 pm, rHH= 74.51 pm.
Transition state can be confirmed by examining its eigenvalue of hessian(one negative and one positive) and interneclear distance vs time plot (no oscillation)
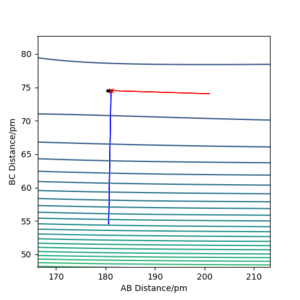
Report the activation energy for both reactions.
Energy at transition state is -433.980 kJ.mol-1
Energy of reactants for F + H2 and H + HF using the same procedure as H + H2 system. As transition state has been located, energy of reactants can be found by importing mep.
The initial condition of the system was slightly displaced from transition state with zero momentum:
rFH+ 1 pm = =182.2 pm, rHH = 74.51 pm, steps was set at number(1000),size(0.3). Last geometry energy (rFH=239.62 pm, rHH=74.02 pm) was recorded at -435.003 kJ.mol-1
Activation energy for F + H2 reaction : -433.980 kJ.mol-1-(-435.003 kJ.mol-1)= 1.023 kJ.mol-1
rFH -1 pm = =180.2 pm, rHH = 74.51 pm, steps was set at number (1000),size (0.3). Last geometry(rFH=92.00 pm, rHH=274.29 pm) energy was recorded at -560.485 kJ.mol-1
Activation energy for H + HF reaction : -560.485 kJ.mol-1-(-435.003 kJ.mol-1)= 125.482 kJ.mol-1
In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. Explain how this could be confirmed experimentally.
From the reactants to products, the small vibrational and tranlational energy will give rise to large vibrational and translational energy. Energy will release in two ways:
<1> vibrational kinetic energy of products
<2> translational kinetic energy of products
Both of them will generate heat( vibrational energy will release as vibratioanl relaxation which given off infrared radiation as heat).Bond calorimetry could measure the total heat release as it will cause temperature rises of outer system.
Chemiluminescence techique can be used to monitor the emission of infrared radiation form the sample.
The other method is to monitor the overtone in the IR, when system generate the products, in this reaction, HF, most of population is in the vibrational ground state, while some is in the 1st vibrational excited state. So it is expected to see the intense 0 to 1 transition, and 1-2 overtone located in higher wave number but lower intensity. 0 to 1 transition will be more intense, while intensity of overtone will decrease when taking snapshot overtime.
there is even a fancy 'application'. Fdp18 (talk) 11:07, 9 May 2020 (BST)
Discuss how the distribution of energy between different modes (translation and vibration) affect the efficiency of the reaction, and how this is influenced by the position of the transition state.
Polanyi principle states that translational energy is more effective than vibrational energy in the reaction with early barrier.
Exothermic reaction(early transition state): F + H2, the reactants with lower vibrational energy could result in high possibility of reactive trajectory.
From graph a,b,c, as the vibrational energy(Higher HH momentum) given to reactants increase, the ease of reactive trajectory will decrease.
Endothermic reaction(late transition state):H+HF, the reactants with higher vibrational energy could result in high possibility of reactive trajectory.
From graph e,f,g, as the vibrational energy(Higher HF momentum) given to reactants increase, the ease of reactive trajectory will increase.
Very nice report that shows good understanding. You could have added a reference section, but that's ok since the references are given in the text. Fdp18 (talk) 11:11, 9 May 2020 (BST)