MRD:ceh15
Exercise 1
How Minima and Transition Structures are Related
The gradient of the potential energy surface is 0 at any minimum and at a transition structure. The transition state is a minimum, since it is part of a valley or trough in energy through which the reaction progresses, however, it is also the maximum point along the reaction path. This is classified as a saddle point, and has a first differential of 0 and second differential of 0. I think you've understand the chemistry part but not really the maths part. For that you need to look at the second partial derivative test if you are interested. I've got a video here. link. --Sw2711 (talk) 12:39, 11 May 2018 (BST)
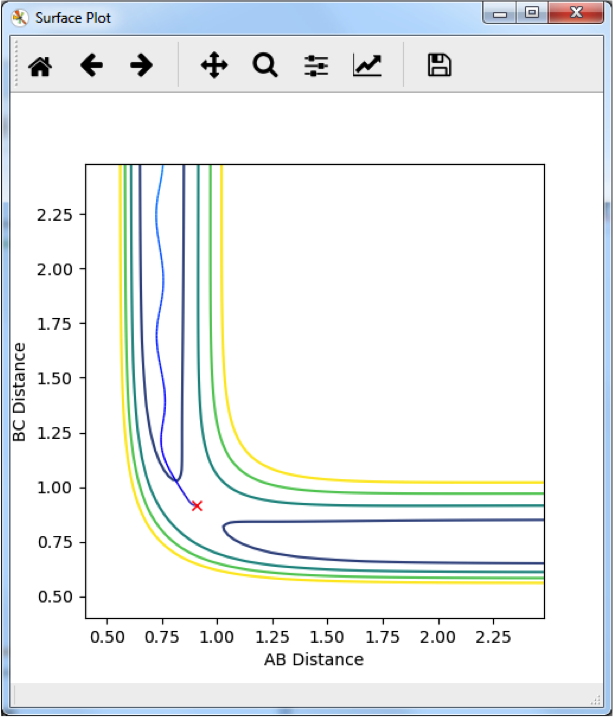
Finding the H2 + H → H + H2 Transition Ttate State--Sw2711 (talk) 13:21, 11 May 2018 (BST)
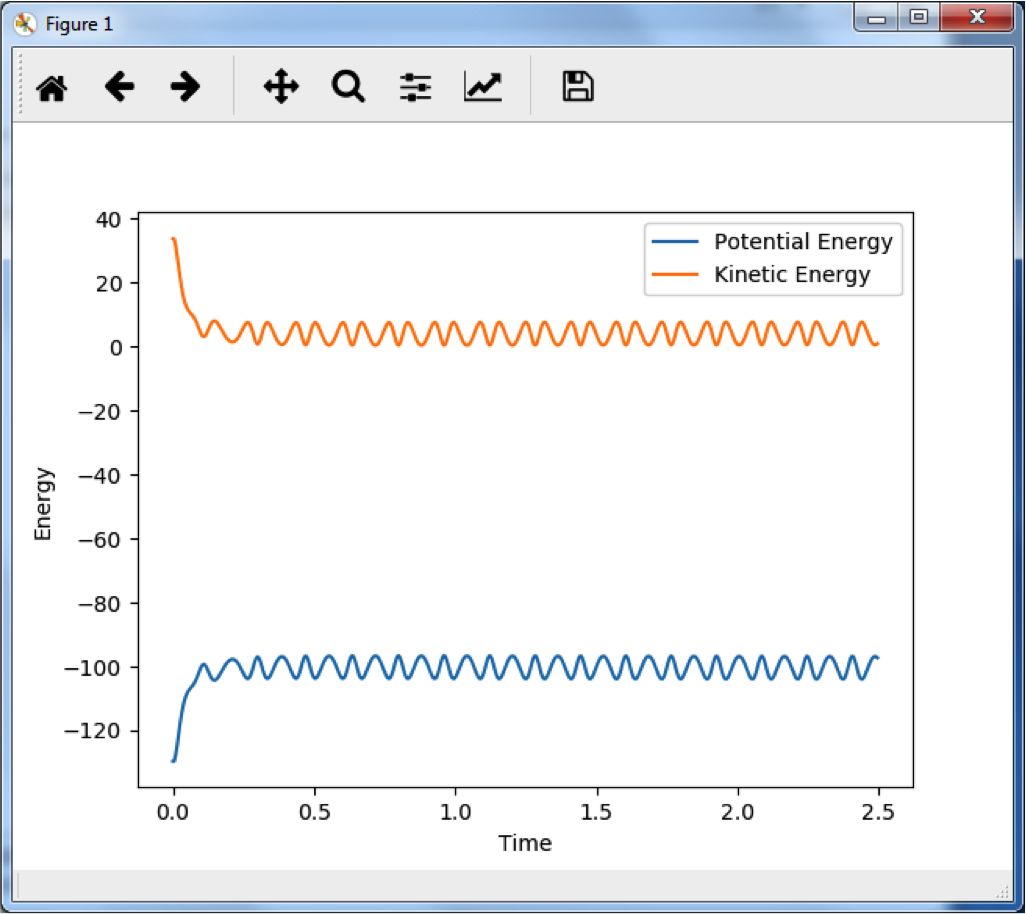
This graph shows the change in internuclear distance over time. Since there is no change, and no momentum was given to the particles in the initial condition, the particles must not be moving along the reaction pathway and we can say that this is the transition state. This occurs at r1 = r1 = 0.9075 Å.
Good--Sw2711 (talk) 13:19, 11 May 2018 (BST)
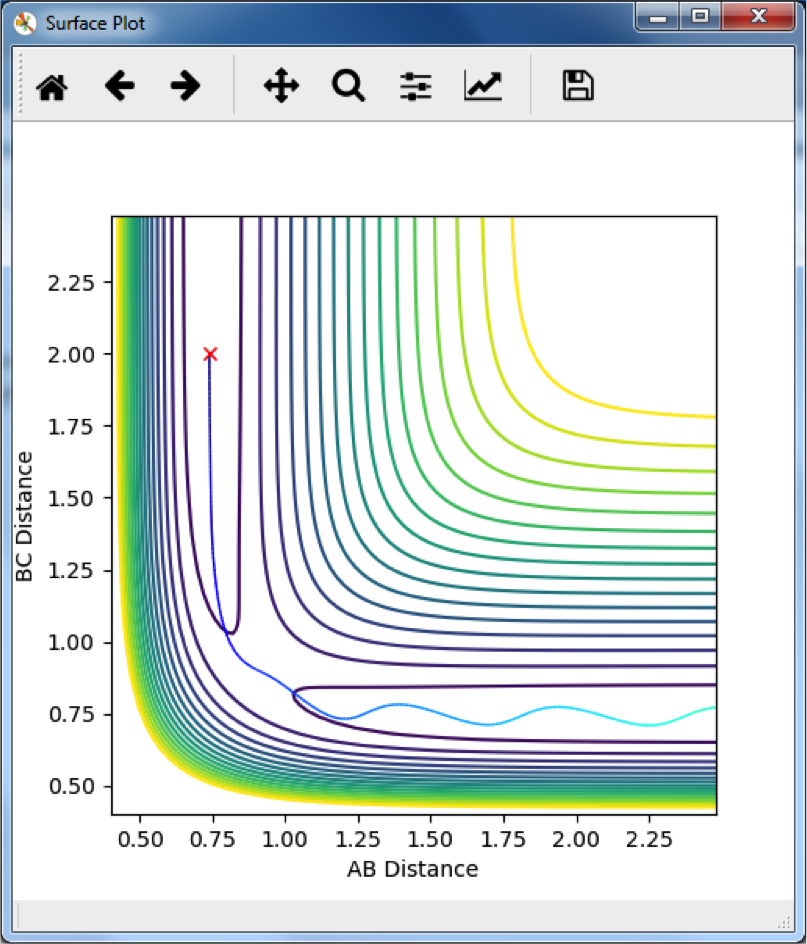
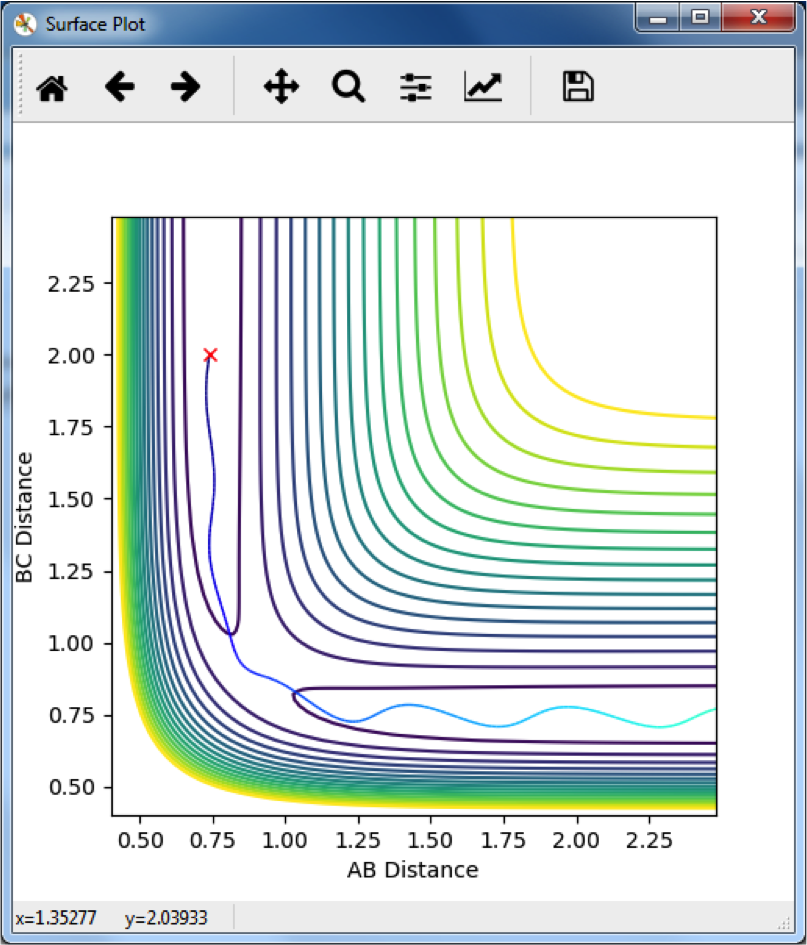
Comparing MEP and Dynamic Trajectories
The MEP follows a path along the minimum energy pathway from the transition state. The dynamic appears to snake across the contours, since it remains at the same energy levels, and so can move up the high energy sides of the reaction pathway. Yeah. ‘Snake’ But what does it actually mean? Did you notice anything about the momentum?--Sw2711 (talk) 13:24, 11 May 2018 (BST)
Reactive and Unreactive Trajectories
| Trajectory Label | p1 | p2 | Total Energy /kcalmol-1 | Reactive? |
|---|---|---|---|---|
| A | -1.25 | -2.5 | -99.018 | Yes |
| B | -1.5 | -2.0 | -100.456 | No |
| C | -1.5 | -2.5 | -98.956 | Yes |
| D | -2.5 | -5.0 | -84.956 | No |
| E | -2.5 | -5.2 | -83.416 | Yes |
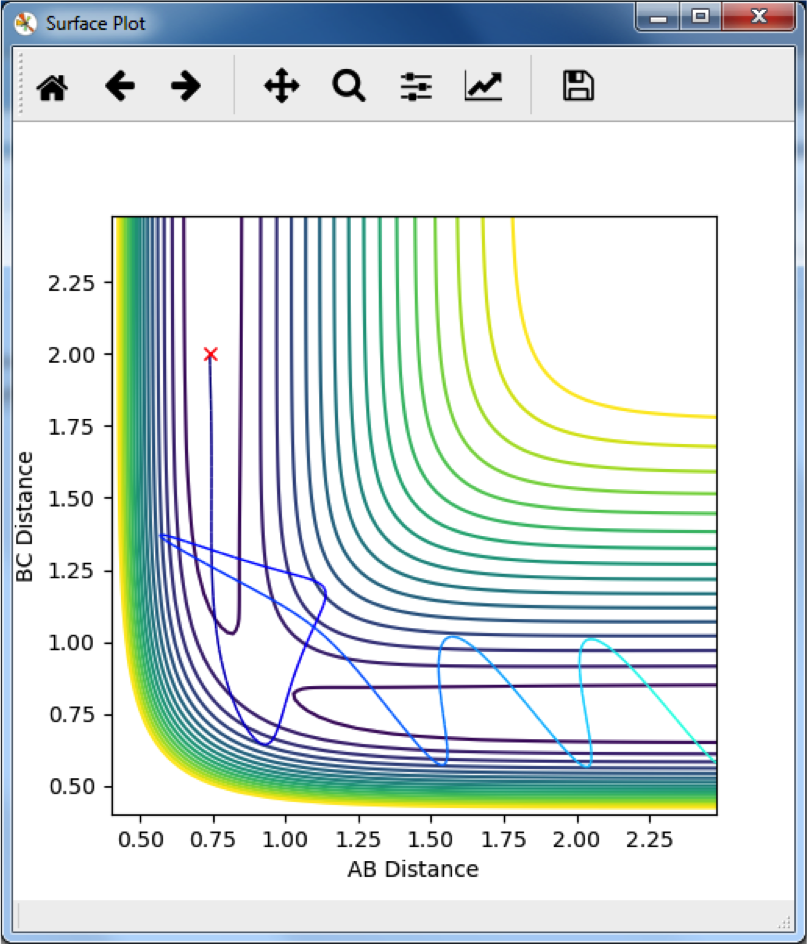
A
The reactants proceed along the minimum reaction pathway, with AB bonded and C approaching, make it over the transition state, where the AB bond breaks and BC bond forms. After the transition state, BC is in a higher vibrational state than AB was, and so we see an oscillation of the BC distance, corresponding to bond vibration. Interesting observation. Why do you think it looks like weaker bond vibration at the beginning then?--Sw2711 (talk) 13:34, 11 May 2018 (BST)
B
Here the bond is already in a high vibrational state, and the AB bond vibrates. The reactants do not cross the transition state, and fall back down the reaction pathway.
Do you mean ‘because the bond is in a high vibrational state, the reactants do not cross the TS?’ I think in your final figure in Polanyi’s Rules, you’ve seen a vigorous vibration, but it still passes the TS. Why is that? In this case, however, it is really just because it doesn’t have enough energy to pass the TS. --Sw2711 (talk) 13:35, 11 May 2018 (BST)
C
Here the AB bond vibrates, and has enough momentum to cross the transition state, where the trajectory is similar to that of A
Good--Sw2711 (talk) 13:35, 11 May 2018 (BST)
D
Here the system has very high energy, but the energy is distributed in a manner that does not lead to the formation of reactants. Although the trajectory does cross the transition state, it recrosses back again, and so the collision is unproductive.
Good--Sw2711 (talk) 13:35, 11 May 2018 (BST)
E
Here the system has very high energy, and the energy is distributed in a manner that does lead to the formation of reactants. Although the trajectory does recross the transition state, back to reactants, it crosses back again, and so products are ultimately formed
Good--Sw2711 (talk) 13:35, 11 May 2018 (BST)
Assumptions of Transition Theory Transition State Theory--Sw2711 (talk) 13:36, 11 May 2018 (BST)
- Atoms behave according to classical mechanics.
- Reactants follow a path along the minimum energy pathway.
- Products cannot recross the transition state, once they have crossed once, they have reacted.
You are missing half of the question. So how does this theory predictions compared to your tests above? --Sw2711 (talk) 13:36, 11 May 2018 (BST)
Exercise 2
Energetics
The HF + H → H2 + F reaction is endothermic. The graph below shows the energy difference between the transition state (at t=0) and the oscillating products. I think this is not true. What you are saying or showing is that the Potential Energy of the transition state is lower than the oscillating products. --Sw2711 (talk) 13:48, 11 May 2018 (BST)
Since the HF + H → H2 + F reaction is endothermic, it follows that the reverse reaction, the H2 + F → HF + H reaction, is exothermic. This suggests that the HF bond is stronger than the HH bond, it has a higher bond dissociation energy, i.e. it takes more energy to break it.
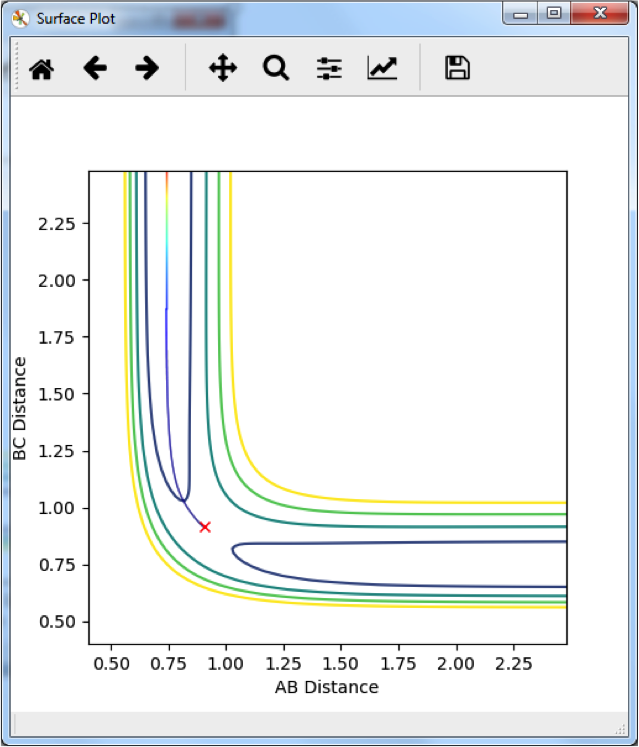
Locating the H2 + F → HF + H Transition State
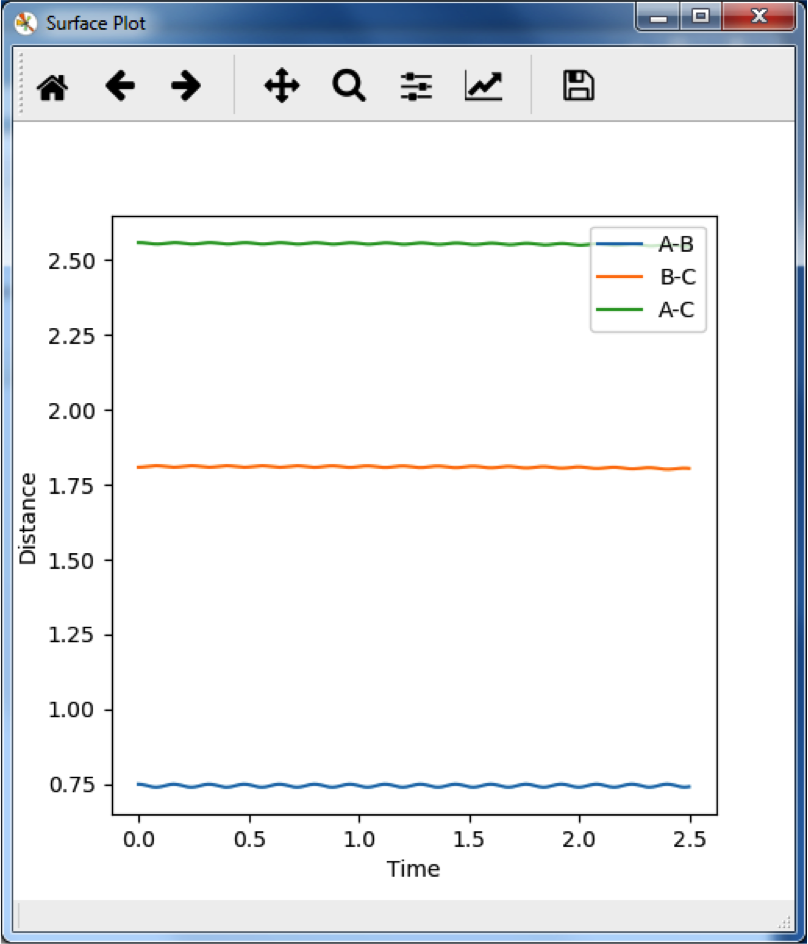
This graph shows the change in internuclear distance over time. Since there is no change beyond slight oscillation, and no momentum was given to the particles in the initial condition, the particles must not be moving along the reaction pathway and we can say that this is the transition state. This occurs at rHH = 0.75 Å and rHF = 1.8082 Å. Just to be precise, slight oscillation is close to a TS. At a TS, we expect no oscillation, no movement at all. So this is NOT the TS, this is just close to the TS. --Sw2711 (talk) 13:49, 11 May 2018 (BST)
Activation Energy
The activation energy of both reactions was found by taking the value of the potential energy of the system at the transition state, and at a point far enough down the reaction pathway that the change in potential energy became negligible, and subtracting one from the other as shown below:
H2 + F → HF + H
EA = ETS - Eground = -103.742 - -104.02 = 0.278 k.cal.mol-1
HF + H → H2 + F
EA = ETS - Eground = -103.742 - -134.025 = 30.283 k.cal.mol-1
We need some evidence for both calculations. Where did you get those numbers from. --Sw2711 (talk) 13:52, 11 May 2018 (BST)
Reactino Dynamics Reaction--Sw2711 (talk) 17:07, 11 May 2018 (BST)
- The potential energy released during this reaction becomes kinetic energy, which manifests in the vibrational energy of the H-F bond and the translational energy of the other hydrogen.
- This could be confirmed experimentally by measuring the temperature of reaction.
Yes, how? I need more explanations on that. --Sw2711 (talk) 13:52, 11 May 2018 (BST)
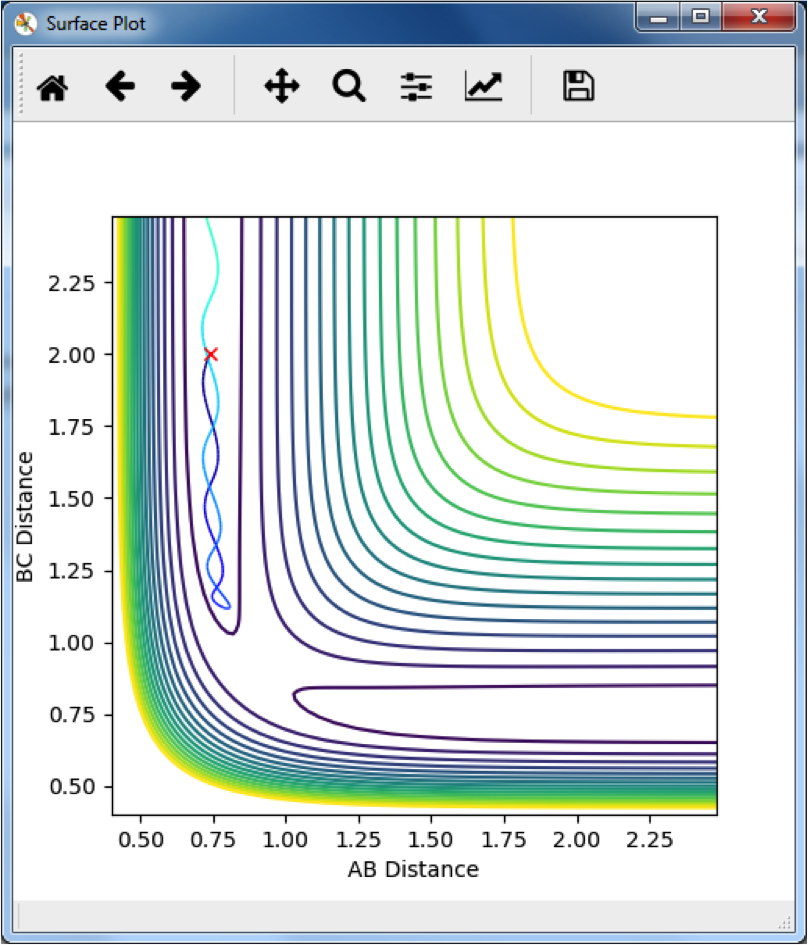
Polanyi's Rules
Polanyi's rules take into consideration two types of energy, vibrational and translational. The rules state that for a reaction with an early transition state (the type that will occur for a highly exothermic reaction with a small energy of activation), it is best to have a large amount of translational energy, and that for a late transition state, it is best to have a large amount of vibrational energy.
The HF + H → H2 + F reaction is highly endothermic, and has a late transition state. This means it requires a large amount of vibrational energy, as can be seen in the reaction profile below: Good--Sw2711 (talk) 13:53, 11 May 2018 (BST)