MRD:cd1618
Questions and Answers
Ng611 (talk) 18:46, 22 May 2020 (BST) Overall: 3/5 - Your answers, whilst mostly correct, were not nearly detailed enough. See the individual comments below for examples.
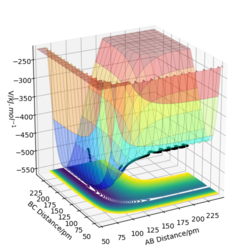
1.On a potential energy surface diagram, how is the transition state mathematically defined? How can the transition state be identified, and how can it be distinguished from a local minimum of the potential energy surface?
A saddle point is a point on the potential surface that the slope in orthogonal directions are zero and is not a local minimum or maximum. It can be identified by finding the first-order saddle point on potential energy surface. To differentiate saddle point from a local minimum, second partial derivative test can be done. Taking two orthogonal curves on potential surface both orthogonal to z axis, if the product of the second derivatives of the is negative, then it is a saddle point. However for a local minimum, the product of second derivatives and the curvature are positive, which indicates the energy goes up in all directions.
Ng611 (talk) 18:38, 22 May 2020 (BST) What are the two othogonal directions on the PES you take the second derivatives with respect to? A diagram or some equations would be helpful.
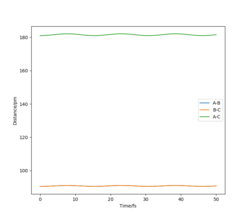
2.Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” plot for a relevant trajectory.
rts= 91 pm All atoms in this system are hydrogen atoms. In this case, a symmetric transition state is expected, the Internuclear Distances vs Time graph confirmed that the distances between AB and BC are the same.
Ng611 (talk) 18:40, 22 May 2020 (BST) I still see minor oscillations in your distances vs time graph. What does that tell you about the TS position?
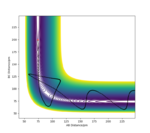
3.Comment on how the mep and the trajectory you just calculated differ.
Dynamic calculation makes trajectory vibrate more at the valley, because it considers the vibration of the bond. Minimum energy path calculation resets momenta to zero in each step, so there is no bond vibration, the trajectory is more like a smooth curve.
Ng611 (talk) 18:40, 22 May 2020 (BST) Some trajectories would have been useful here.
4.Complete the table above by adding the total energy, whether the trajectory is reactive or unreactive, and provide a plot of the trajectory and a small description for what happens along the trajectory. What can you conclude from the table?
Even with high enough energy, a trajectory is not always reactive. Right amount of energy is required for the reaction to happen. The system can go beyond the transition state and recross the energy barrier back to reactant.
5.Given the results you have obtained, how will Transition State Theory predictions for reaction rate values compare with experimental values?
TST will overestimate reaction rate, because it assumes any trajectory with kinetic energy higher than activation energy will be reactive, but from the simulation, it can be found that even with higher energy can recross the transition state and goes back to reactant. So experimentally, the rate will be lower than the estimated value.
Ng611 (talk) 18:40, 22 May 2020 (BST) Good answer. What are the other assumptions of TS theory though?
6.By inspecting the potential energy surfaces, classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved?
Exothermic for F + H2 and endo thermic for H + HF, this indicates that the bond strength for H-F is higher than H-H. So breaking a weak H-H bond to form a strong H-F bond will be exothermic and vice versa
Ng611 (talk) 18:44, 22 May 2020 (BST) You are absolutely correct, but what features of the TS would support your answer? If, for example, you only had the TS to go on, how could you confirm the exo/endothermicity of the reaction?
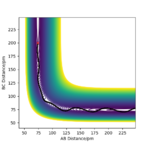
7.Locate the approximate position of the transition state.
F-H distance 181 pm H-H distance 75 pm
Ng611 (talk) 18:44, 22 May 2020 (BST) How did you confirm this was the TS?
8.Report the activation energy for both reactions.
-433.940 kJmol-1 for transition state 0.066 kJmol-1 for F + H2 It is very close to 0 and hard to determine. 125.367 kJmol-1 for H + HF
Ng611 (talk) 18:44, 22 May 2020 (BST) Again, how was this calculated?
9.In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. Explain how this could be confirmed experimentally.
The energy of bond forming is transferred to the vibration of F-H bond, shown in the Momenta vs Time diagram. The Momentum of F-H experienced a huge increase when the system goes over the transition state. In the experiment, this can be confirmed by infrared chemiluminiscence. Also, the internal vibration will be converted to translational energy and transferred to the surrendering molecules.
Ng611 (talk) 18:44, 22 May 2020 (BST) IR chemiluminesence is absolutely correct, well done. I'm unsure for your second part though. How would this help?
10.Discuss how the distribution of energy between different modes (translation and vibration) affect the efficiency of the reaction, and how this is influenced by the position of the transition state.
In the exothermic process examined here, F + H2, translational energy is more effective in overcoming the activation energy barrier. This can be confirmed by calculation, the total kinetic energy is much lower when more energy in the system is the translational mode. Because the early transition state can be more easily overcomed by translational energy.[1] This is realated to the position of transition state.
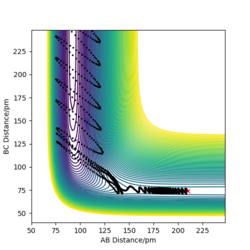 p1= -1 g.mol-1.pm.fs-1 p2= 0.6 g.mol-1.pm.fs-1 Ek= +1.486 kJmol-1
p1= -1 g.mol-1.pm.fs-1 p2= 0.6 g.mol-1.pm.fs-1 Ek= +1.486 kJmol-1
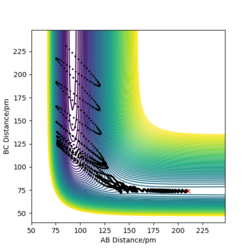 p1= -1.05 g.mol-1.pm.fs-1 p2= 0 g.mol-1.pm.fs-1 Ek= +0.580 kJmol-1
p1= -1.05 g.mol-1.pm.fs-1 p2= 0 g.mol-1.pm.fs-1 Ek= +0.580 kJmol-1
In the endothermic process, FH + H, vibrational energy is more effective to promote reaction. The initial condition with higher vibrational energy requies less total kinetic energy to reach TS.
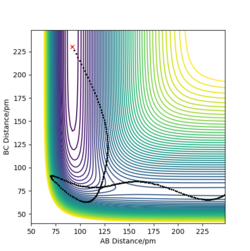 p1= -0.2 g.mol-1.pm.fs-1 p2= -19 g.mol-1.pm.fs-1 Ek= +357.221 kJmol-1
p1= -0.2 g.mol-1.pm.fs-1 p2= -19 g.mol-1.pm.fs-1 Ek= +357.221 kJmol-1
 p1= 13.2 g.mol-1.pm.fs-1 p2= -2.4 g.mol-1.pm.fs-1 Ek= +129.145 kJmol-1
p1= 13.2 g.mol-1.pm.fs-1 p2= -2.4 g.mol-1.pm.fs-1 Ek= +129.145 kJmol-1
Ng611 (talk) 18:45, 22 May 2020 (BST) A good set of examples. Well done!
References
- ↑ Polanyi, J. C. "Some Concepts in Reaction Dynamics." Science 236.4802 (1987): 680-90.