MRD:caw116
Exercise 1: H + H2 system
Minima and transition structures in potential energy surfaces
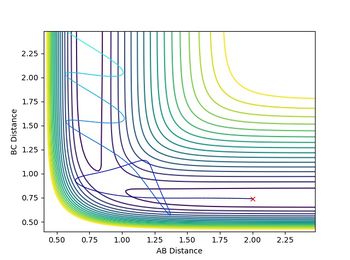
The graph giving potential energy surfaces of a given reaction arises from a function with respect to two individual variables, namely the different bond distances AB and BC. Here, a relative minimum/maximum can only be achieved when the derivatives with respect to both variables equal to 0.
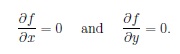
A transition state however occurs when the potential energy surface leads to a saddle point. At this point both derivatives with respect to the different variables equal to 0, however one of them resembles a maximum, the other one a minimum in the curvature. Which one of the two occurs can be examined by expanding the Taylor expansion used to determine the first derivative up to second order.
If (fxxfyy-fxy2) < 0 then a saddle point can be observed.
Location of the transition point
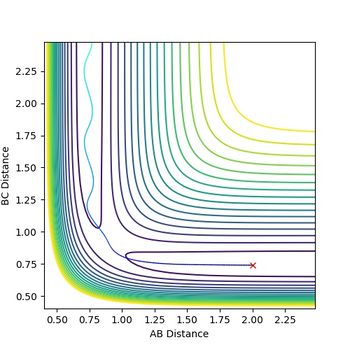
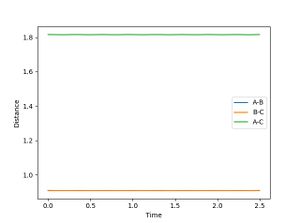
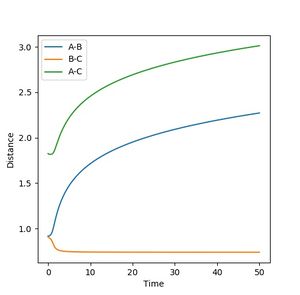
The transition state has to be observed on a reactive trajectory between the reactants and the products. One can roughly estimate this position by looking at the contour plot. The initial momenta are then set to 0, so that the molecule does not have enough energy to escape the minimum of the potential well. The distance versus time plot gives a flat line for both distances being equal to 0.908, which means that the molecule does not move and the transition state has been reached.
Jas213 (talk) 18:28, 13 May 2018 (BST) Good that you described your method of how you found the transition state.
The difference between mep and and dynamics
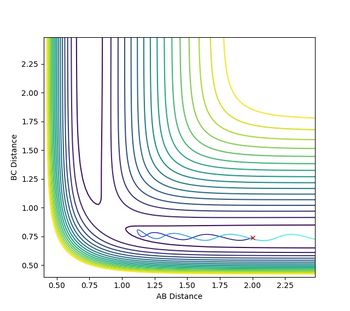
The plot of the mep follows the minimum energy path of the reaction. The velocity of the molecule is continuously set back to zero throughout the reaction, so that the inertia of the molecule is not taken into account anymore. This can be observed in the plot itself by changing the settings from dynamics to mep and comparing the oscillation behaviour in both cases. There is no oscillation behaviour observed in the mep plot.
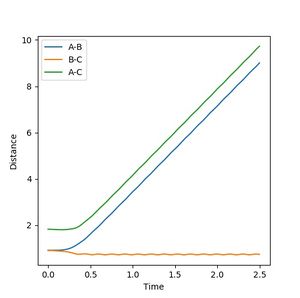
Reactive and unreactive trajectories
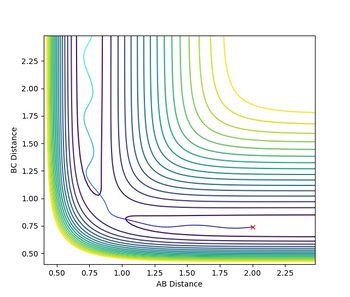
Jas213 (talk) 18:30, 13 May 2018 (BST) What do you conclude from this table? What did you notice regarding the difference in kinetic energy? The last two cases are examples of re-crossing. At the start it would have been nice, if you stated your conditions used.
Transition State Theory
The Theory separates reaction systems into two different regions, the reactant-space, in which the starting material has not reacted yet and the product space, which is entered after reaction occurred. The transition state is defined as the border between these 2 spaces, which the reactants have to overcome to give the products. |As soon as this border has been overcome and the reactants have given the products, the state cannot be reversed and the products cannot enter the reactant space again.
This theory is based on the assumption that the atoms obey the Born-Oppenheimer approximation and that quantum-tunneling effects do not occur. The atoms of the starting material also have to have energies being distributed according to the Boltzmann distribution, which presumes that the system had enough time at the beginning to equilibrate.[2]
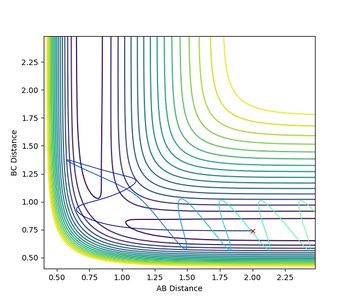
However, considering the obtained calculations for reaction 4 shown in the picture above, it can be seen that the reactants are actually able to enter the product space and then dissociate to the starting material again, so that the theory cannot be applied here. This is because the calculations performed by the program are assuming isolation of the reactant system, while the theory takes the surrounding and the possible energy exchange with neighbouring molecules into account so that equilibrium is achieved.
Exercise 2: F-H-H system
PES inspection
The reaction F + H2 ⇌ H + HF is in equilibrium, where the the reaction to the right is the exothermic one, releasing energy, and the backwards reaction is the endothermic one, consuming energy. [3] This is due to the HF bond being higher in energy (-565 kJ/mol) than the H2 - bond (-432 kJ/mol) [4], so that more energy is needed to break the HF bond than that is gained by forming the H2 bond. Therefore, this reaction is endothermic and the backwards reaction is exothermic.
Jas213 (talk) 18:36, 13 May 2018 (BST) Very clear explanation of the overall reaction above.
The transition state can approximately observed at the following settings
Atom A: F
Atom B: H
Atom C: H
AB distance: 1.81
BC distance: 0.7456
Both momenta are set to 0.
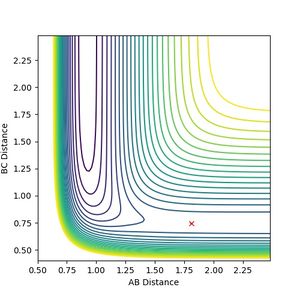
Jas213 (talk) 18:33, 13 May 2018 (BST) How did you find the transition state? What method did you use?
For the activation energy, change the calculation type to MEP and displace the AB distance from equilibrium at the transition state, so that the molecules are forced to go in either direction, products or reactants.
To find the activation energy, the calculation type is changed to MEP and the AB distance is displaced from the equilibrium at the transition state, so that either of the reactant/product spaces is depicted.
The reaction F + H2 --> FH + H is the exothermic one so that due to Hammond's postulate the transition state resembles the reactants more than the products. The transition state therefore occurs at the beginning of the reaction, which means that to obtain the activation energy, the value for the AB-distance has to be displaced to a higher value. +0.10
(increase steps to 10000)
Energy of the transition state = -103.752
Energy of products = -104.005
Activation energy of the reaction = 0.253
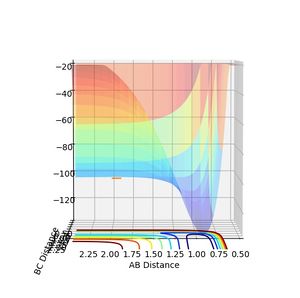
The endothermic reaction however proceeds in the other direction and goes up the potential well. The transition state distance therefore has to be displaced to a lower value. -0.10
Energy of the transition state = -103.752
Energy of products = -131.047
Activation energy of the reaction = 27.295
Jas213 (talk) 18:36, 13 May 2018 (BST) Where are your units?
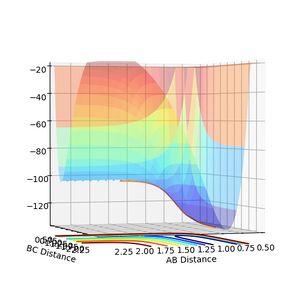
Jas213 (talk) 18:50, 13 May 2018 (BST) Good that you illustrated activation energies using MEP plots. However, very misleading figure annotation, going from H2 to HF is exothermic, not endothermic.
Reaction dynamics
A reactive trajectory was found to be
Atom A : F
Atom B : H
Atom C : H
AB distance = 1.914
BC distance = 0.7456
AB momentum = -1.5
BC momentum = 1.0
Jas213 (talk) 18:40, 13 May 2018 (BST) How did you find this TS? How do you know it is the actual transition state? Where is your MEP to prove it?
Considering the momentum vs. time plot, it can be seen that the reaction results in an increased and vigorous oscillation of the resulting molecule. It can therefore be concluded that the reaction energy released during the exothermic process is converted into vibrational energy of the molecule. Furthermore, the energy vs. time plot shows an increased oscillation in the kinetic energy of the newly formed molecule.
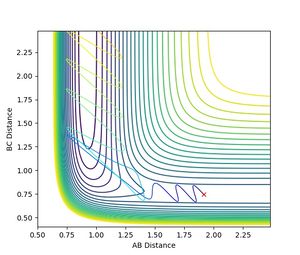
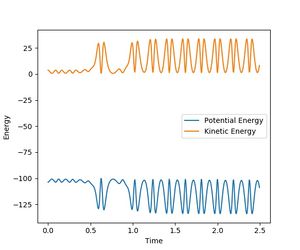
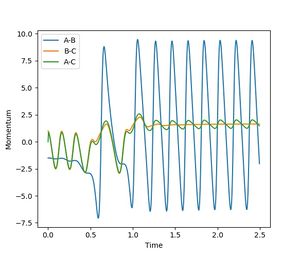
Investigating on the impact of the changing AB-momentum
Atom A : F
Atom B : H
Atom C : H
AB distance = 1.914
BC distance = 0.74
AB momentum = -3 to 3
BC momentum = -0.5
As soon as the AB momentum reaches 0, no more reaction can be observed. Increasing the value in the positive direction only results in a change of the oscillation strength of the AB-bond.

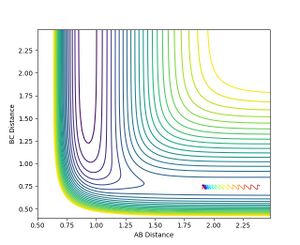
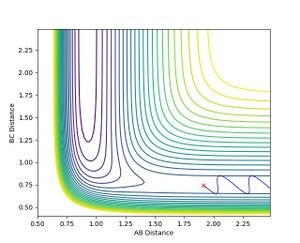
Jas213 (talk) 18:41, 13 May 2018 (BST) Figure 11 and 12 don't make sense. Your reactions are going the wrong way on the PES.
Polanyi's rules
The Polanyi rules discuss the effect of the different motions, vibrational and translational, on a reaction. It is found that vibrational motion is more efficient at promoting a late transition state, which is energetically closer to the products, whereas translational motion is better at supporting a transition state which resembles the reactants more than the products. [5]
As the F + H2 reaction shows an exothermic reaction pathway, the transition state occurs at an early point during the reaction is must therefore be promoted by translational motion. This motion is resembled by the momentum corresponding to the molecules which initially are further apart (in this case the AB momentum), as this momentum is responsible for the strength of the collision.
The backwards reaction, however, which is endothermic and shows a late transition state, is therefore promoted by a high initial vibrational energy of the starting material. This energy is represented by the momentum of the initially bonded molecule.
Thus, it depends on the reaction monitored which momentum leads to a promotion of which reaction.
The reaction examined above corresponds to the endothermic one, which explains why an increase in the AB-momentum results in no reaction.
Jas213 (talk) 18:48, 13 May 2018 (BST) Good that you mentioned Polanyi rules.
References
1. R.L Jacobs, 2016, "Mathematics and Physics for Chemists 1", Imperial College London, p.48-49
2. T.Bligaard, J.K. Norskov, Chemical Bonding at Surfaces and Interfaces, 2008, "Chapter 4 - Heterogeneous Catalysis
3. J.C. Polanyi, D.C. Tardy, 1969, J. Chem. Phys., Vol.51 "Energy Distribution in the Exothermic Reaction F + H2 , and the Endothermic Reaction HF + H", p.255-321
4. http://www.wiredchemist.com/chemistry/data/bond_energies_lengths.html, accessed 04.05.18, 13:56
5. Z. Zhang et al, J.Phys. Chem. Lett., 2012, "Theoretical Study of the Validity of the Polanyi Rules for the Late-Barrier Cl + CHD3 Reaction", Vol. 3 (23) p.3416–3419