MRD:bea01198491
Atom-Diatom Collisions
OVERALL COMMENTS: A good report overall, although you made a major error calculating the transition state energies. 4/5
Exercise 1: H+H₂ System
Dynamics from the transition state region
On a potential energy surface diagram, how is the transition state mathematically defined? How can the transition state be identified, and how can it be distinguished from a local minimum of the potential energy surface?
A potential energy surface diagram maps the potential energy of the total system relative to physical parameters such as r1 and r2. The transition state occurs at a saddle point on the potential energy surface ie. where ∂V(r1)/∂r1 = 0 and ∂V(r2)/∂r2 = 0; ∂²V(r1)/∂r12 < 0, ∂²V(r2)/∂r22 > 0 or vice versa (one of the second derivatives will be negative). The constraints on the second partial derivatives with respect to r1 and r2 differentiate the saddle point from the local minimums of the surface which correspond to the reactants and products, for which ∂²V(r1)/∂r12 > 0, ∂²V(r2)/∂r22 > 0. As both first partial derivatives are equal to 0, the system is stable exactly at the transition state (but unstable with a minute perturbation to either side of the transition state). This means the transition state can be identified as a system which has no kinetic energy.
Good answer. One thing that you haven't done though is defined r1 and r2, are these the rAB/rBC vectors in the programs PES? If not, a diagram of the PES showing the basis vectors of r1 and r2 would be helpful.
Report your best estimate of the transition state position (rts) and explain your reasoning, illustrating it with a “Internuclear Distances vs Time” plot for a relevant trajectory.
If the system is set up in the geometry of the transition state with no initial momentum, then it should remain in the transition state geometry indefinitely and have no kinetic energy. Furthermore, in the symmetrical Ha + HbHc-> HaHb+ Hc system, the distances between the atoms, r1 and r2, must be equal in the transition state, i.e. r1=r2 (this constraint may also prevent the transition state from being confused with the minima associated with reactants/products, where r1>r2 and r2>r1).
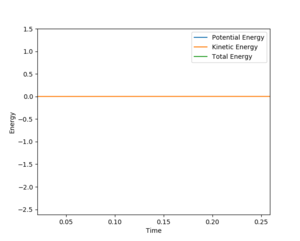
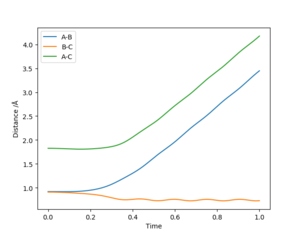
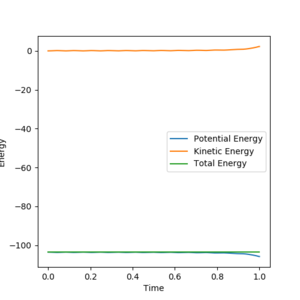
Energy vs time graphs were plotted by repeatedly inputting increasingly accurate values for r1 and r2 (with r1=r2) into Leps Gui, with p1 = p2 = 0 (as there is no kinetic energy there is no momenta), with the values for r1 and r2 improved via trial and improvement until a plot was obtained where the kinetic energy was static at 0 J as seen in figure 1. The internuclear-distance vs time graph was plotted for these r1/r2/p1/p2 values, and showed r1 and r2 to be static and equal at rts = r1 = r2= 0.9077432 Å, i.e. the atoms are stationary as the system is in the transition state, as seen in figure 2.
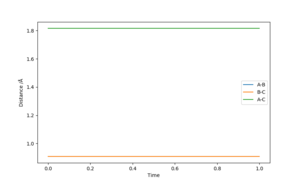
Comment on how the MEP and the trajectory you just calculated differ.
The MEP, figure 3 and 4, and the dynamic trajectory, figure 5, were both plotted with r1 = rts + 0.01 = 0.9177432 Å, r2 = rts = 0.9077432 Å and p1 = p2 = 0. The MEP predicts that under these circumstances the internuclear distances r1 and r2 will both tend to constant values (ie. the atom Ha will move increasingly slowly away from Hb/Hc), while the internuclear distance r2 (really the Hb-Hc bond length) will settle relatively quickly to a constant value (with no vibration). The predictions of Ha's motion becoming infinitely slow, and the absence of vibration in the Hb-Hc bond (i.e. a constant r2 after the collision) is due to the MEP ignoring the inertial motion of atoms.
Good answer! I'd say that you've reported the value of rTS to an excessive number of decimal places (to an accuracy of about 1/50th the width of a single proton)
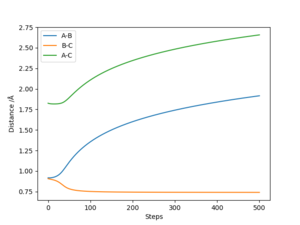
Meanwhile, the dynamic model shows the r1 and r1+r2 internuclear distances increasing by a positive gradient which has some slight sinusoidal variation, i.e. Ha moves away from HbHc at a constant velocity, but the internuclear distances do not increase with a constant gradient because of the Hb-Hc vibration. This Hb-Hc vibration is also seen in the r2 bond length, which varies sinusoidally around a constant value of ~ 0.74 Å. The momenta vs time plot for these initial conditions gives values of p1 = 2.5, and p2 = 1.25 after 1 second.
What would change if we used the initial conditions r1 = rts and r2 = rts + 0.01 instead?
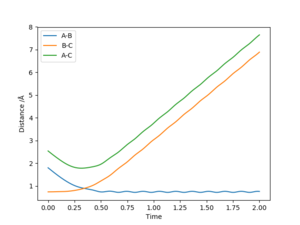
When the initial conditions are reversed, so that r1 = rts+0.01 = 0.9077432 Å, r2 = rts = 0.9177432 Å, p1 = p2 = 0; both the internuclear-distance vs time and momenta vs time plots for r1 exactly resembles that for r2 under the initial conditions, and the plot for r2 exactly resembles that for r1 under the initial conditions. This is because exactly the same sort of collision is occurring, but with Ha colliding with the diatomic HbHc to form a new diatomic HaHb which Hc moves away from, instead of Hc colliding with HbHa forming a new diatomic HcHb which Ha moves away from as occurred under the initial conditions of r1 = rts + 0.01 = 0.9177432 Å, r2 = rts = 0.9077432 Å, p1 = p2 = 0. The Ha + HbHc-> HaHb+ Hc reaction is symmetrical, so when the forwards reaction occurs instead of the reverse then all the plots stay the same and just the atom assignments change.
Set-up a calculation where the initial positions correspond to the final positions of the trajectory you calculated above, the same final momenta values but with their signs reversed. What do you observe?
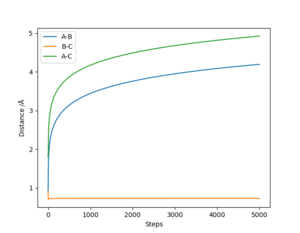
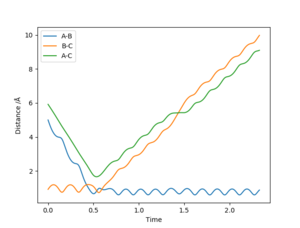
The internuclear distance vs time plot shown in figure 6 was created with the conditions r1 = 0.8 Å, r2 = 0.74 Å, p1 = -2.5, and p2 = -1.25. It shows A approach the diatomic B-C (which is not vibrating), pass through a transition state where r1 = r2 at the intersection of the lines, then A and B remain in a new diatomic which vibrates sinusoidally, with bond length r1 varying around 0.74 Å, while C travels away with a constant velocity. A reaction therefore occurs under these conditions.
Reactive and un-reactive trajectories
Complete the table by adding the total energy, whether the trajectory is reactive or un-reactive, and provide a plot of the trajectory and a small description for what happens along the trajectory. What can you conclude from the table?
| p1 | p2 | Etot (3 d.p.) | Reactive? | Description of the dynamics | Figure |
|---|---|---|---|---|---|
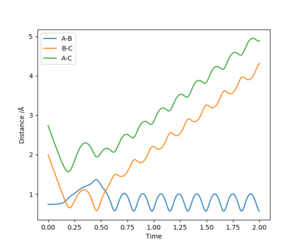
| -1.25 | -2.5 | -99.018 | Yes | C approaches AB, A leaves BC (small vibration). | 7 |
| -1.5 | -2.0 | -100.456 | No | C approaches AB, C repelled by AB (small vibration) before any significant interaction occurs. | 8 |
| -1.5 | -2.5 | -98.956 | Yes | C approaches AB, A leaves BC (small vibration). | 9 |
| -2.5 | -5.0 | -84.944 | No | C approaches AB, large distortion occurs with a barrier recrossing so that C is expelled by the TS and leaves AB (large vibration). | 10 |
| -2.5 | -5.2 | -83.390 | Yes | C approaches AB, large distortion occurs and A leaves BC (large vibration). | 11 |



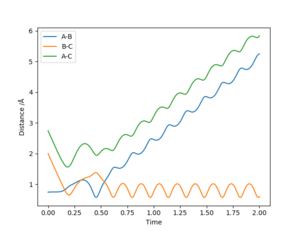
We can conclude that the hypothesis stands - i.e. all trajectories for initial conditions r1 = 0.74 Å, r2 = 2.0 Å, -1.5 < p1 < -0.8, and p2 = -2.5 are reactive. Meanwhile, trajectories were tested with p2 values other than -2.5, and some were reactive and some were un-reactive (and this did not seem to correspond to having larger or smaller p2 values than -2.5). Furthermore, whether the reaction occurs or not appears to be independent of p1, although there are larger vibrations in the diatomic products for reactions occurring with higher p1 values (and greater distortion in the transition states).
State what are the main assumptions of Transition State Theory. Given the results you have obtained, how will Transition State Theory predictions for reaction rate values compare with experimental values?
Transition State Theory states that, with sufficient (activation) energy, colliding atoms/molecules will form a high energy unstable complex known as a transition state, which can decay back into the original reactants or go on to form new products. The transition state is assumed to exist in a dynamic equilibrium with the reactants, so that the conversion of reactants to products can be re-written as a two-step process of reactants (with sufficient activation energy) forming a transition state and then this transition state subsequently decaying to form products. The transition state will take on the vibrational/rotational/electronic/translational energy of the reactants, eg. the linear triatomic A..B..C produced from the collision of C and AB (with sufficient energy) will have stretching and bending vibrational modes, however Transition State Theory does not implement selection rules for the transfer of these quantised energies. Furthermore, Transition State Theory assumes atoms/molecules follow classical mechanics, particularly in the requirement of the products to have a minimum activation energy in order to form a transition state upon their collision - which is disputed by the quantum tunnelling of small particles described by quantum mechanics.
Transition State Theory would predict that all reactant collisions with sufficient energy would lead to a reaction occurring. This does not agree with the barrier crossing in our results, as seen in figure 10, where the system crosses the transition state region but goes on to reform the products anyhow (perhaps due to a quantum mechanical restriction from a selection rule not being met?). Transition State Theory therefore has limitations despite its use in modelling elementary chemical reactions, and the prediction that the rate of a reaction will increase if the activation energy is more readily available (i.e. through higher temperatures) may be disputed by experiment.
Good answer and most things are correct, although I think you could make your answer slightly clearer in a few ways: (1) would predict that all reactant collisions with sufficient energy would lead to a reaction occurring - more specifically, any system that reaches the TS goes on to form products (2) perhaps due to a quantum mechanical restriction from a selection rule not being met I doubt this, it is more likely that the first product formed has so much energy that it is unstable (and so dissociates); after the first recrossing, the energy of the system is distributed in such a way that the resulting products are far more stable. (3) prediction that the rate of a reaction will increase if the activation energy is more readily available (i.e. through higher temperatures) - Given your results, this is reasonable, do you expect to see multiple recrossings at low temperatures then (hint: yes you should)? If so then what can you say about the accuracy of TS theory over all conditions?
Exercise 2: F - H - H system
PES inspection
By inspecting the potential energy surfaces, classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved?
F + H2: exothermic - because the polar covalent F-H bond formed is much stronger than the purely covalent H-H bond broken (the large electronegativity difference between F and H causes an electrostatic attraction which reinforces the covalent bond between the two atoms) so more energy is released than absorbed.
H + HF: endothermic - because the energy released by the formation of the weaker H-H bond is not sufficient to compensate for the energy absorbed in the breaking of the strong F-H bond, so energy is absorbed from the environment to make up the difference.
Locate the approximate position of the transition state.
At the transition state, all the atoms will be instantaneously stationary, so there will be no kinetic energy in the system and the total energy will be equal to the potential energy. Hammond's Postulate states that the transition state will most resemble in structure the reactants or products which it is closest in energy to. As the transition state for the reaction F + H2 -> H + HF is closest in energy to the high energy reactants (as the reaction is exothermic), the transition state structure will be most similar to F + H2, with r1 (the F-H internuclear-distance) being longer than the average F-H bond length of 0.92 Å by a greater extent than r2 (the H-H internuclear-distance) is longer than the average H-H bond length of 0.74 Å.
Through trial and improvement to find the energy vs time plot with kinetic energy nearest to zero, the internuclear-distances in the transition state were found to be r1 = rts1 = ~1.70 Å and r2 = rts2 = ~0.77 Å, while the momenta are of course p1 = p2 = 0. The energy vs time plot for these initial conditions (which should remain constant!) is shown in figure 12, where it is possible to see the slightly sinusoidal kinetic and potential energy curves (due to the diatomic vibrating), which also deviate from their average values after a significant period of time.
Report the activation energy for both reactions
An MEP, as shown in figure 13, was run with the initial conditions r1 = rts1 + 0.01 = 1.70 + 0.01 = 1.71 Å, r2 = rts2 = 0.77 Å, p1 = p2 = 0; and with 1000 steps of size 0.01. The energy MEP gave a constant total energy for the transition state of -133.9870. It was certain the transition state had been reached as the kinetic energy was constant at 0.
An energy vs time MEP was used to find the reactants' total energy (ie. for F + H2 with r1=3, r2 =0.74, p1=p2=0) as -103.9903. Therefore, the activation energy for F + H2 -> FH + H is -133.9870 - (-103.9903) = -29.9967.
An energy vs time MEP was used to find the products' total energy (ie for FH + H with r1 = 0.92, r2=3, p1=p2=0) as -133.9974. Therefore, the activation energy for FH + H -> F + H2 is -133.9870 - (-133.9974) = 0.0104. As it is unlikely that the transition state is lower in energy than the products, this very small positive value is likely due to the limits of the precision of the program.
These calculations are incorrect. What is the physical meaning of a negative activation energy? Also, using your chemical intuition, does it make sense that the magnitude of the activation energy for F+H2 is higher than FH+H?

Reaction dynamics
Identify a set of initial conditions that results in a reactive trajectory for the F + H2, and look at the “Animation” and “Momenta vs Time”. In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. Explain how this could be confirmed experimentally.
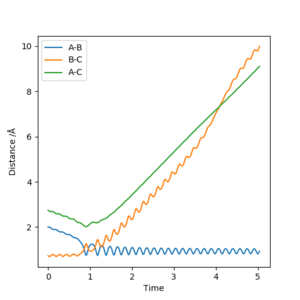
A reactive trajectory for F + HbHc -> FHb + Hc is achieved for the initial conditions r1 = 2, r2 = 0.74, p1 = -1.25, and p2 = -1.5, as shown by the internuclear-distance vs time plot seen in figure 14, with r1= the F-Hb internuclear-distance and r2=the Hb-Hc internuclear-distance. The animation and momenta vs time graph show far greater bond vibrations in the HF diatomic product than in the H2 reactant, due to the energy 'released' by the exothermic reaction (from the higher potential energy of the reactants) being converted to the kinetic energy of the products (so the total energy in the system remains constant). The conversion of potential energy to kinetic energy (i.e. vibrational, rotational and translational motion) could be measured as an increase in temperature during the course of a reaction. An increase in the diatomic vibrational energy could be measured through Raman or IR spectroscopy, and the decrease in potential energy from reactants to products can be seen in the products having a lower dissociation energy than the reactants.
Setup a calculation starting on the side of the reactants of F + H2, at the bottom of the well rHH = 0.74, with a momentum pFH= -0.5, and explore several values of pHH in the range -3 to 3 (explore values also close to these limits). What do you observe? Note that we are putting a significant amount of energy (much more than the activation energy) into the system on the H - H vibration.
Internuclear-distance vs time graphs were plotted for r1 = 2 Å, r2 = 0.74 Å, p1 = -0.5, and -3 > p2 < 3, in order to discover if a reaction of F + HbHc -> FHb + Hc would occur when the system was given these initial conditions. The activation energy required to reach the transition state was exceeded in every system, so all of the interactions reached transition states, and many then lead to the formation of products. However, the transition states reached in some systems collapsed to reform the product, eg. for p2 = -2.9, -1.5, 1, 2.2, involving barrier recrossing (which may be due to selection rule restrictions). Furthermore some systems, eg. with p2 = 0.4, 1.1, continually returned to the transition state from repeat barrier recrossing, so that the three atoms remain in a triatomic system in a constant state of flux. The reaction took place at the range limit of p2 = 3.000, however not at p2 = -3.000 (although it did for p2 = -2.998), and also took place when p2 = 0 (after several barrier crossings and re-crossings). Whether a reaction occurs (or not) does not appear to be linked to the size (or sign) of p2. The values of p2 which prevent the transition state from forming products due to barrier recrossing(s) appear to be fairly random (and perhaps due to restrictions from selection rules).
When a reaction does occur, the vibrational energy of the diatomic product HF is seen to be much greater than the vibrational energy of the diatomic reactant H2. As seen from the animation (and internuclear-distance vs time graphs) the HF bond vibration is caused by the movement of the (light) Hb atom - hence the F-Hc bond length does not show such large vibrations as the Hb-Hc bond length. The size of the diatomic product's vibrations do not appear to be proportional to the initial momentum of the H2 diatomic bond p2 - for example, they appear to be roughly the same size for p2 = 0 as for p2 = 2.9 as for p2 = -2.8.
For example, figure 16 shows the trajectory for a system with initial conditions r1 = 2 Å, r2 = 0.74 Å, p1 = -0.5, and p2 = 0.1, which is reactive.
For the same initial position, increase slightly the momentum pFH = -0.8, and considerably reduce the overall energy of the system by reducing the momentum pHH = 0.1. What do you observe now?
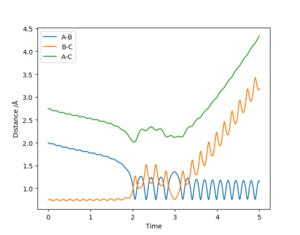
When the system is given the initial conditions r1 = 2 Å, r2 = 0.74 Å, p1 = -0.8, and p2 = 0.1, the reaction takes place following a brief barrier crossing, as shown in figure 17. The higher p1 value increases the speed at which the H atom leaves the diatomic HF product in comparison to under the previous initial conditions (seen in figure 16). There are also less barrier crossings in the formation of the products, which makes the rate of reaction around twice as fast as with the previous conditions.

Setup initial conditions starting at the bottom of the entry channel, with very low vibrational motion on on the H-F bond, and an arbitrarily high value of pHH above the activation energy (an H atom colliding with a high kinetic energy).
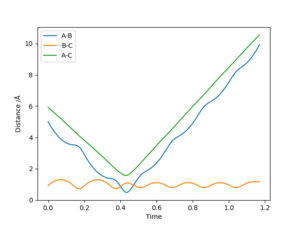
We now consider the reaction Ha + HbF -> HaHb + F, where r1 is the Ha-Hb internuclear-distance and r2 is the Hb-F internuclear-distance. Figure 18 gives the internuclear-distance vs time plot with initial conditions r1 = 5 Å, r2 = 0.92 Å, p1 = -10, and p2 = -0.01, and shows an un-reactive trajectory due to a barrier crossing.
Try to obtain a reactive trajectory by decreasing the momentum of the incoming H atom and increasing the energy of the H-F vibration.
Figure 19 shows the internuclear-distance vs time plot from initial conditions r1 = 5 Å, r2 = 0.92 Å, p1 = -8, and p2 = -0.1, giving a reactive trajectory.
The cases studied are an illustration of Polanyi's empirical rules. Discuss how the distribution of energy between different modes (translation and vibration) affect the efficiency of the reaction, and how this is influenced by the position of the transition state.
Polanyi's empirical rules state that for reactions with early transition states, a large difference in the translational energies of the reactants is best in achieving the required activation energy, while for reactions with late transition states a large vibrational excitation of the reactants is best for achieving the required activation energy.
As discussed earlier, the reaction F + H2 -> HF + H is exothermic so has an early transition state, while its reverse, H + HF -> H2 + F, is endothermic so has a late transition state. In both reactions, the value of p1 indicates the translational speed at which the atom reactant approaches the diatomic reactant, as none of the kinetic energy indicted by this momentum value can be stored as vibrational energy (and the amounts stored by rotation are always very small in comparison). Meanwhile, the value of p2 indicates the vibrational energy of the diatomic (through bond stretching/bending) as any translation energy the diatomic has will only act to increase/decrease the value of p1 because only the relative translational speeds are considered.
In the reaction F + H2 -> HF + H, initial conditions with high p1 translational energies resulted in reactions occurring, no matter the values of p2 (apart from some disallowed values most likely due to selection rules). As this is the exothermic reaction with the early transition state, this fits with Polanyi's empirical rules.
Meanwhile, in the reaction, H + HF -> H2 + F, initial conditions with a higher p2 vibrational energy resulted in a previously un-reactive reaction occurring, despite a lower value of p1. As this is the endothermic reaction with the late transition state, the fits with the latter of Polanyi's empirical rules.
Good!
