MRD: SA
Introduction
Exercise 1: H + H2 system
On a potential energy surface diagram, how is the transition state mathematically defined? How can the transition state be identified, and how can it be distinguished from a local minimum of the potential energy surface?
The saddle point of a potential energy surface is the point where the transition state is located. For the reaction of H with H2 at the transition state the distances AB and BC are equal. Any infinitesimally small change from the transition will cause the system to leave the saddle point (TS) towards either the the reactants or products. Correct, but you should mention the derivative of energy being zero and second derivatives being positive or negative depending on direction. Also, you haven't distinguished the TS from local minima. Pu12 (talk) 21:56, 16 May 2019 (BST)
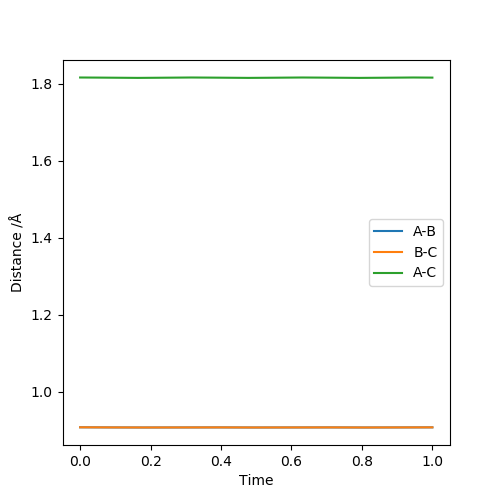
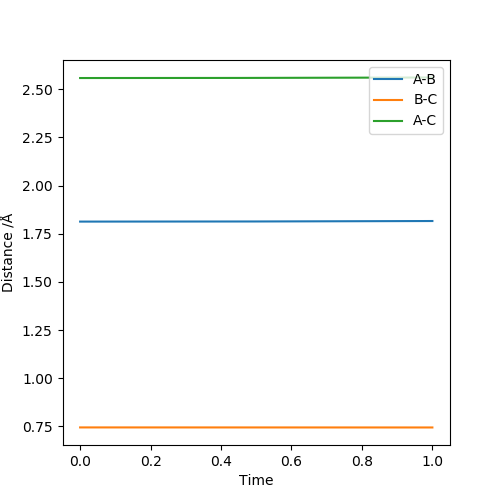
Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” plot for a relevant trajectory.
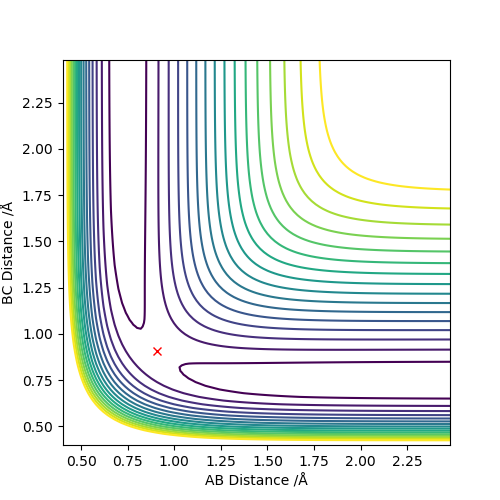
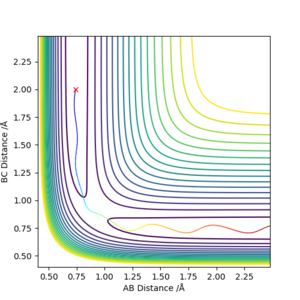
The transition state positions for the two AB and BC are approximately 0.908 Å, since on the contour plot, the trajectory is non-existent. Moreover, the A-B and B-C distance remains constant, as would be expected in the transition state.
Comment on how the mep and the trajectory you just calculated differ.
The main difference in those is velocity. For the MEP the velocity is set to zero after each step. This results in the momenta always being zero for the MEP calculation, whereas for the dynamic calculation the momenta changes.
What about vibration?. Pu12 (talk) 21:56, 16 May 2019 (BST)
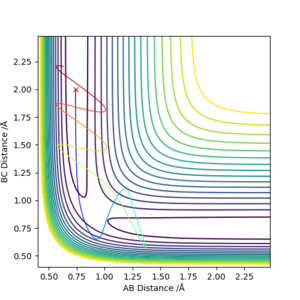
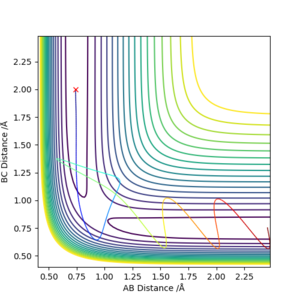
Complete the table above by adding the total energy, whether the trajectory is reactive or unreactive, and provide a plot of the trajectory and a small description for what happens along the trajectory. What can you conclude from the table?
State what are the main assumptions of Transition State Theory. Given the results you have obtained, how will Transition State Theory predictions for reaction rate values compare with experimental values?
There are several assumptions that are made by the transition state theory:
1. Quantum-tunneling effects are assumed negligible and the Born-Oppenheimer approximation is invoked.
2. The of the particles involved have energies that are Boltzmann distributed, this is usually satisfied if the system has thermally equilibrated.
3. Once reactants have crossed the transition state they must form products, they cannot turn around and reform the reactants.
4. The reactants are in constant equilibrium with the TS structure.
You haven't anwsered part of the question, what about the comparison of TS theory predictions to experimental values?. Pu12 (talk) 21:56, 16 May 2019 (BST)
Exercise 2: F - H - H system
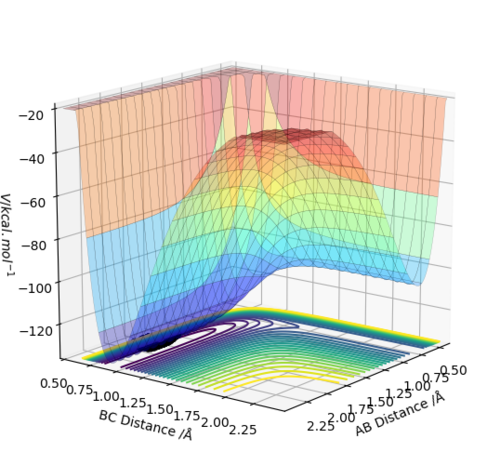
By inspecting the potential energy surfaces, classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved?
Distance
H-F: 2 Å
H-H: 0.74 Å
Momentum
H-F: -0.5 kgms-1
H-H: 2.90 kgms-1
exothermic (products are lower in energy than the reactants)

Distance
H-H: 2 Å
H-F: 0.92 Å
Momentum
H-H: -0.5 kgms-1
H-F: 2.55 kgms-1
endothermic (reactants are lower in energy than the products)
All correct, but again, you haven't anwsered part of the question - what about bond energies?. Pu12 (talk) 21:56, 16 May 2019 (BST)
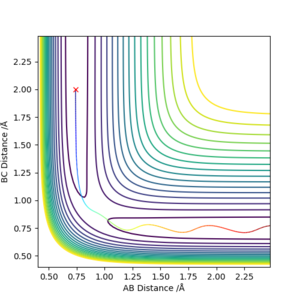
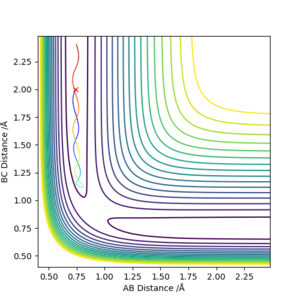
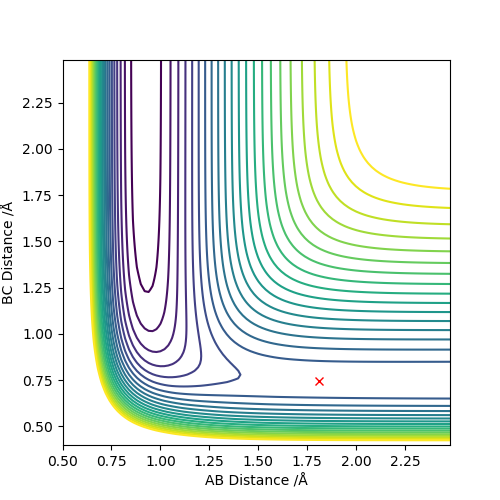
Locate the approximate position of the transition state.
F-H 1.810 Å
H-H 0.746 Å
This is the approximate position of the transition state as in the contour graph below there is only one point and there is no trajectory and the distances remain constant over time.
Good Pu12 (talk) 21:56, 16 May 2019 (BST)
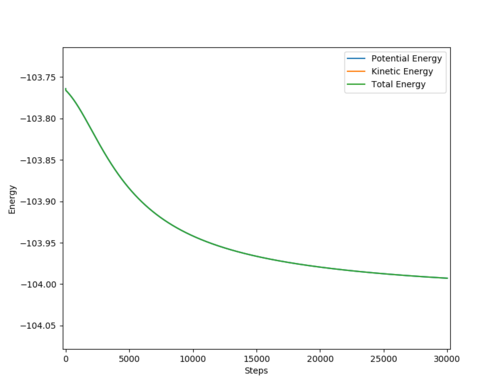
Report the activation energy for both reactions.
The activation energy is the difference between the energy of the transition state and the reactants.
TS energy: - 103.753
F + H2 model
Activation energy = -103.753 - (-103.992) = +0.239 kcal/mol
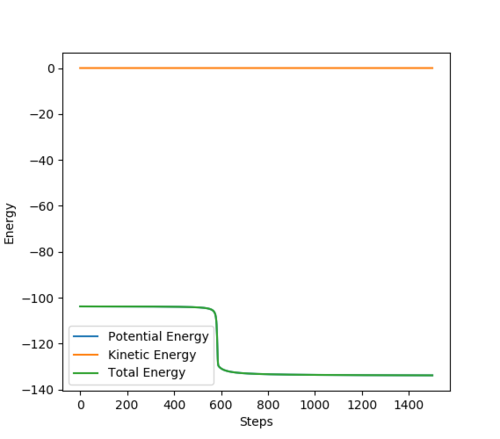
HF + H model
Activation energy = -103.753 - (-133.580) = +29.827 kcal/mol
In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. Explain how this could be confirmed experimentally.
The data above suggests that the H-F bond is stronger than the H-H bond. The reaction of breaking the H-F bond requires energy to be put into the system whereas for the other reaction energy is released.
When bonds are broken energy is absorbed and when bonds form energy is released. This question is more about a discussion of translational energy being converted to vibrational energy. Also, you didn't say how your idea could be confirmed experimentally. Pu12 (talk) 21:56, 16 May 2019 (BST)
In case of the reaction HH + F --> HF + H
breaking the H-H bond requires a small amount of energy, but formation of the H-F bond releases a bigger amount of energy, so overall the reaction results in a release of energy.
(Vice versa for HF + H)
Discuss how the distribution of energy between different modes (translation and vibration) affect the efficiency of the reaction, and how this is influenced by the position of the transition state.
Polanyi's rules state that for a reaction:
- if there is an early barrier, kinetic energy is effective in overcoming this barrier, whereas vibrational energy is ineffective; - if there is a late barrier, vibrational energy can effectively overcome the barrier, but kinetic energy is ineffective.
That means, that for a late TS, as the ones in endothermic reactions, vibrational energy has the larger effect on efficiency, and for an early TS, such as in exothermic reactions, translational energy will have the larger effect on the efficiency.
Good, but please reference. Pu12 (talk) 21:56, 16 May 2019 (BST)
References
This report has good displays of understanding, however falls behind due to incomplete answers Pu12 (talk) 21:56, 16 May 2019 (BST)