MRD:ZH3615
Investigation of the molecular reaction dynamics of different systems under various initial conditions
Zehua Hu Answers correspond to the blue questions in the script are underlined in this write-up
Abstract
Reaction dynamics of triatomic systems H-H-H and F-H-H were investigated, transition states and reactivity under various initial conditions were determined from contour plot and intermolecular momenta/time plot by using MATLAB.
Introduction
Potential energy surface together with the reaction trajectory of a triatomic system can be generated using matlab by plotting B-C bond distance against A-B bond distance, where the atom species, the initial momentum and initial bond distances are given. The reaction is reactive when the reaction trajectory passes through the transition state from product region to the product region without coming back, where the transition state is the saddle point in the surface plot with highest potential energy and first/second derivative = 0. Hence position state of various conditions can be studied and activation energy can be obtained. Significance of translational energy and vibrational energy in early barrier and late barrier reaction can be obtained from study of surface plot, internuclear distance vs time plot and internuclear momenta vs time plot using dynamics and MEP calculation respectively.
Result and Discussion
1. H-H-H system
The total gradient of the potential energy surface is ∂V (r1)/∂r1=0 both at the minimum and transition structure. Minimums and transition structure can be distinguished by calculate second derivative ∂V (r2) /∂r2, ∂V (r2) /∂r2=0 for transition structure and ∂V (r2) /∂r2>0 at minimums, as the transition structure is the saddle point.
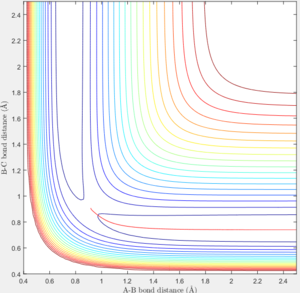
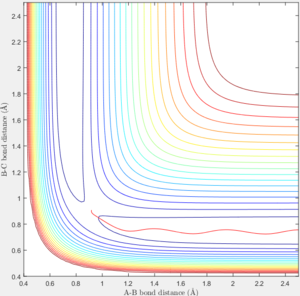
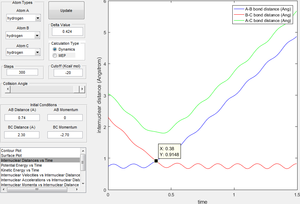
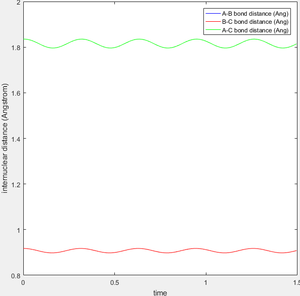
The transition state is defined as the maximum on the minimum energy path linking reactants and the products. [1] Approximate internuclear distance corresponds to the transition state is obtained by plotting internuclear distance vs time as shown in figure 1a, however, slight fluctuation is observable when r1=r2=0.9148 as shown in figure 1b. The optimum internuclear distance during the transition state (rts) is estimated to be 0.90775 as shown in figure 1c by applying bisection method, which is the accurate intercept of bond distance curves.
 |
 |
 |
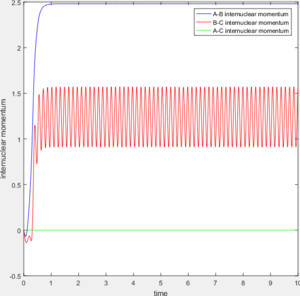
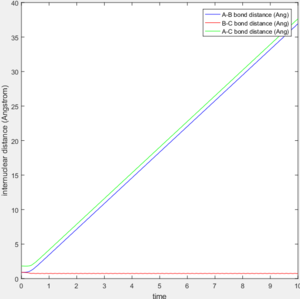
For comparison between MEP and trajectory calculated, value of r1, r2, p1 and p2 is adopted to be 0.90, 0.91, 0 and 0 separately. The difference between the mep and the trajectory calculated is shown below in table 1. As the MEP neglects the internuclear momenta, absence of fluctuation in internuclear distance/momenta is observable in the MEP plots.
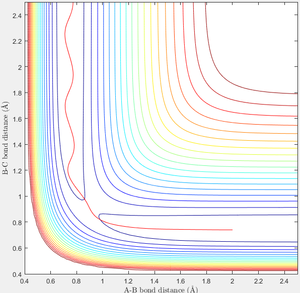
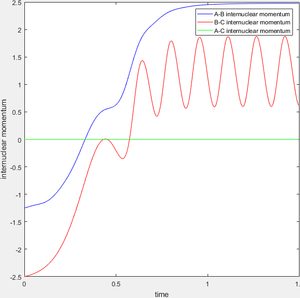
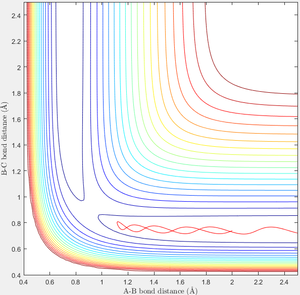
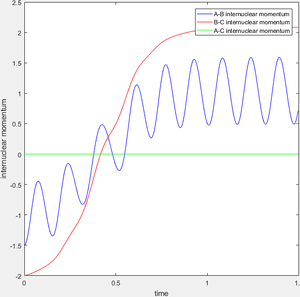
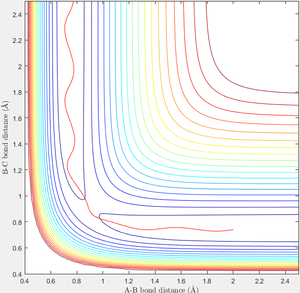
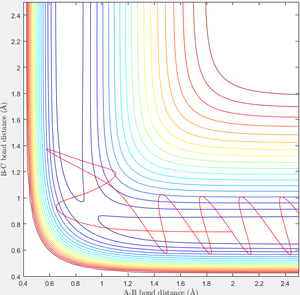
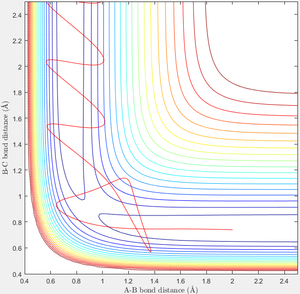
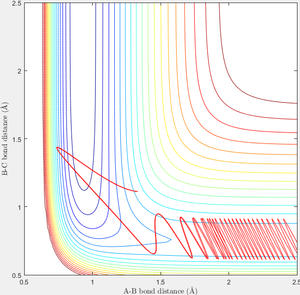
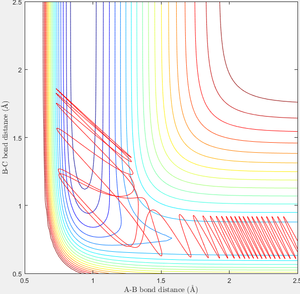
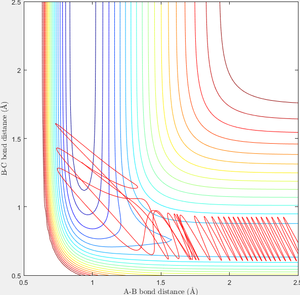
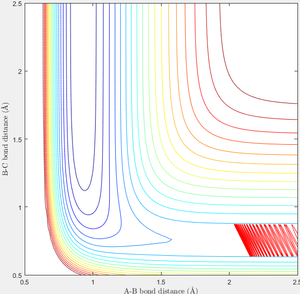
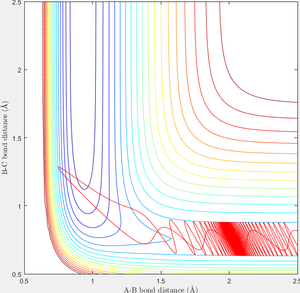
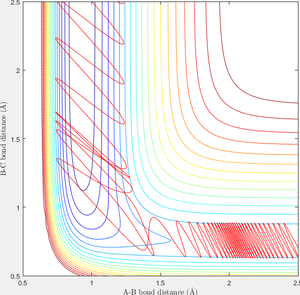
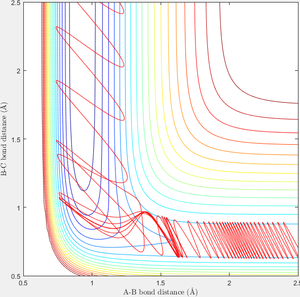
When r1=0.74 and r2=2.0 and the initial momenta are the values shown in table 2, the reactive/unreactive information of the trajectory and the screenshots correspond to every initial conditions are shown below in table 1 and figure 2-6.
The transition state theory assumes that the reactant system would proceed to the product system once it passes through transition state region, which is not true according the results obtained above. In condition 4 and 5, both trajectory passes through the transition state region, the trajectory comes back to reactant and goes back to product in condition 5 while the trajectory stays in reactant region after coming back from product region. Hence the transition state theory predictions for reaction rate values would be higher compared to experimental values as some successful reaction predicted by the transition state theory would not actually react and hence actual reaction rate is far less that theoretical prediction.
2. F-H-H system
As H-F is stronger than H-H bond, energy is released when H-F bond is formed.F+H2 reaction is exothermic and H+HF reaction is endothermic as shown in figure 7.
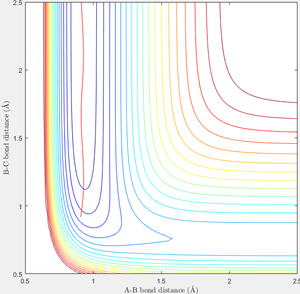
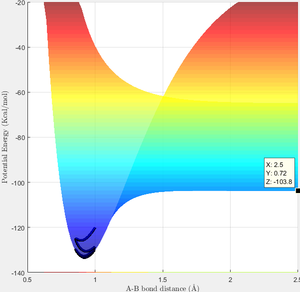
Approximate position of transition state (i.e. maximum between reactant region and product region according to RGB colour) is located using the similar approach as above, which is (1.82, 0.74, -103.7) as shown in figure 8. Hence the transition state is estimated to be at r1=1.82 and r2=0.74.
 |
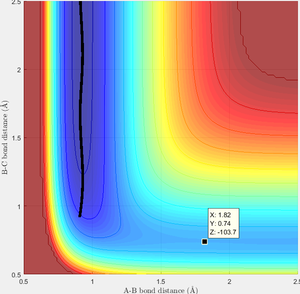 |
As activation energy is the energy barrier between transition state and reactant, by locating the two minimums in the surface plot (i.e. the potential energy of reactant and reactant), activation energy of F+H2 reaction and H+HF reaction is obtained to be 0.1 Kcal/mol and 30.2 Kcal/mol separately as shown in figure 9 and 10. However, this calculation is based on the approximation that the lowest point of this excerpt plot is the full surface plot, and its value equals to the value at infinite distance.
 |
 |
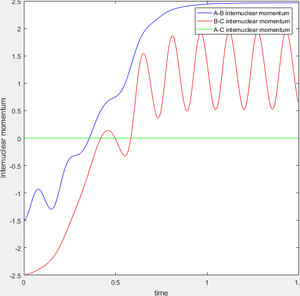
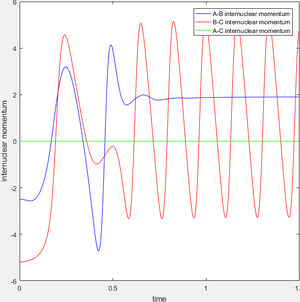
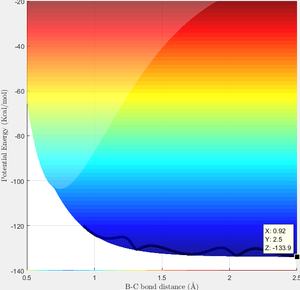
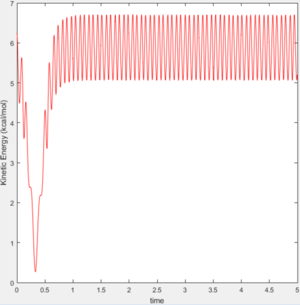
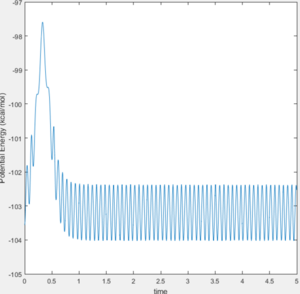
During the reaction of F atom with hydrogen molecule, the attacking particle loses its kinetic energy as shown in figure 11, which converted to potential energy i.e. vibrational energy to overcome the energy barrier as shown in figure 12 and figure 13. As parameter temperature is involved in the partition function of vibrational energy, the mechanism can be confirmed by carrying out F-HH colliding experiment with same amount of reactants but different initial momentum, and measuring temperature/ethaply change of each reactions.
 |
 |
 |
|---|
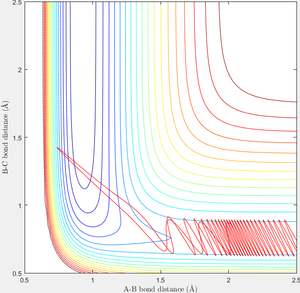
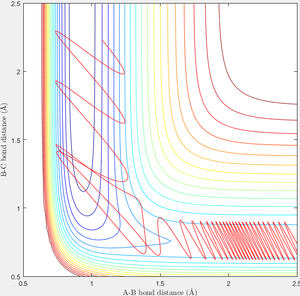
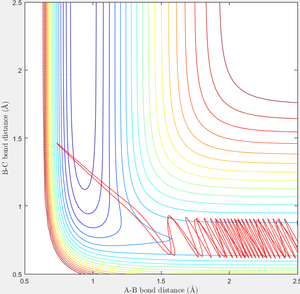
In order to find a set of reactive trajectory of F-HH reaction, initial conditions of F-H-H system are set as PF-H=-0.5, rF-H=2.5, rH-H=0.74, steps=2000. Experiment with various values of PH-H from -3 to 3 are carried out and following results are obtained as shown in table 3.
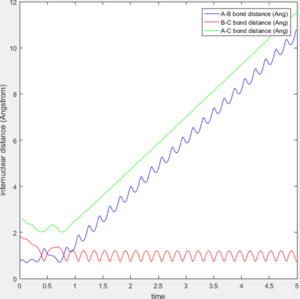
When initial condition is changed to PH-H=0.1, PF-H=-0.8, the following contour plot is obtained. Small vibrational energy can be observed before the formation of F-H bond and cleavage of H-H bond, larger vibrational energy is observable afterwards as excessive energy released from the formation of F-H converts to vibrational energy.
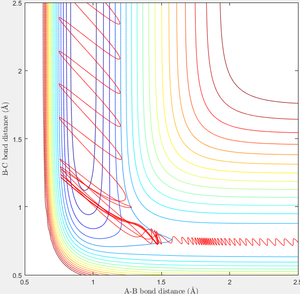
For late barrier reaction i.e. barrier at exit channel, which is the case for H+HF reaction, Polanyi's empirical rules suggest that vibrational energy is more efficient to promoting the reaction compared to translational energy. [2] The position of transition state is in entrance channel for exothermic reaction while that is in exit channel for endothermic reaction. T.S. at entrance channel would require certain amount of translational energy to overcomes to energy barrier while vibrational energy becomes more important when T.S. is at the exit channel as higher vibrational energy would be easier for particles to approach each other.
Conclusion
Transition state of triatomic system H-H-H and F-H-H is studied and discussed using internuclear distance vs time and surface plot as references. Difference between MEP and dynamics calculation in these systems with various initial conditions are discussed. Reactivity of systems with different initial conditions is discussed by observing the surface plot together with the reaction trajectory. Energetics of H-HF and F-HH reaction is dicussed and activation energy of both reactions are calculated. Effect of the distribution of energy between translation and vibrational energy on late barrier and early barrier reaction is discussed and further experiment in order to confirm the conservation of energy is suggested.
Reference
[1] Imperial Chemistry Wiki, https://wiki.ch.ic.ac.uk/wiki/index.php?title=CP3MD
[2] Zhang, Zhaojun, et al. "Theoretical study of the validity of the Polanyi rules for the late-barrier Cl+ CHD3 reaction." The journal of physical chemistry letters 3.23 (2012): 3416-3419.