MRD:TTY115 Y3
Transition States and Reactivity
Introduction
A series of Diels Alder reactions along with potential competing reactions were investigated. PES (potential energy surface) calculations were done for all the reactions.
The reactions were all simulated using Gaussian and visualised using GaussView, the software considers all of the atoms participating in the reaction, their geometries and calculates their electronic potential energy considering all degrees of freedom. Degrees of freedom corresponds to the number of normal vibrational modes, which are (3N-5) and (3N-6) for linear and non-linear molecules respectively, where N represents the number of atoms in the molecule. A PES is then calculated by only varying two degrees of freedom.
Nf710 (talk) 00:36, 8 November 2017 (UTC) Not quite correct A PES is visualized in 2D not it is actually a 3N-6 artifact and you can move in the space of the 3N-6 normal modes and vary the energy
In a PES, minima are represented by points along the reaction profile where the first derivative (gradient) is zero and the second derivative (curvature) is positive; at this point, energy increases in both directions, indicating that at this point, stable chemical species are present, which often correspond to reactants and products. The reaction coordinate is given by the minimum energy pathway between two minima which is represented as IRC (Intrinsic Reaction Coordinate). The highest point along the minimum energy pathway is known as the transition state of a reaction which is represented by a maximum in a PES, where the first derivative (gradient) is zero and the second derivative (curvature) is negative; meaning that energy decreases moving in both directions from this point. It should also be noted that at the transition state, one imaginary frequency would be observed.
Nf710 (talk) 00:36, 8 November 2017 (UTC) This is correct but only for the reaction coordinate. the rest of the 3N-4 degrees of freedom are minima
Two simulation methods were employed in these calculations: 1) Semi-empirical PM6 and 2) DFT-B3LYP-631G(d). Intial optimisations were done using the semi-empirical PM6 method, while DFT-B3LYP 631G(d) was used for further and more accurate optimisations. The semi-empirical PM6 method employs the use of experimental data in calculations while DFT B3LYP, as the name suggests, employs Density Functional Theory to run its calculations. IRC calculations use calculated force constants in order to calculate subsequent reaction paths following transition states.
Nf710 (talk) 00:36, 8 November 2017 (UTC) Nice brief intro about the methods. It would have been good if you could have gone into abit more detail here
A typical Diels Alder reaction was investigated in exercise 1, while an inverse electron demand Diels Alder reaction and a hetero diels alder reaction were investigated in exercise 2 and 3 respectively.
Exercise 1

In this exercise, a Diels Alder reaction between butadiene and ethene was investigated. The reactants, product (Cyclohexene) and the transition state were all optimised using the semi-empirical PM6 method. The reaction scheme is shown in figure 1.
MO Diagram

Figure 2 shows an MO diagram for the Diels Alder reaction between butadiene and ethene. The Jmol objects of the MOs representing the reactants butadiene and ethene are presented here. The Jmol objects of the transition states orbitals involving the HOMO, HOMO-1, LUMO, LUMO+1 are presented here.
By comparing these MOs with the MO diagram presented in Figure 2, it can be concluded that the requirements for symmetry is such that only symmetric/symmetric and antisymmetric/antisymmetric interactions are allowed and are observed from the above simulations. It can be seen from Figure 2 and the Jmol objects that butadiene symmetric MOs interacted with ethene symmetric to give the LUMO and HOMO; while antisymmetric MOs of the two reactants interacted to form HOMO-1 and LUMO+1.
Therefore, it can also be concluded that a symmetric/antisymmetric interaction would have an orbital overlap of zero while symmetric/symmetric, antisymmetric/antisymmetric interactions would have non-zero orbital overlaps. It should also be noted that the energy levels of the MOs have to be in close proximity in order to overlap effectively.
(Fv611 (talk) 11:32, 2 November 2017 (UTC) Even though your MO diagram is almost correct (you made a mistake in drawing the butadiene LUMO), you do not correlate the MOs you have calculated with your diagram at all. You call them HOMO-1/HOMO/LUMO/LUMO+1 in your Jmol page but you don't label them the same way in your diagram. Additionally the symmetry and orbital overlap reasoning is the wrong way around: it is the orbital overlap that gives rise to symmetry rules, i.e. A/S linear combinations are not allowed because the orbital overlap would be zero.)
Bond Lengths Analysis
Measurements of the 4 C-C bond lengths in the reactants and the 6 C-C bond lengths in the transition state and the product are recorded in Figure 3 as shown below.

The change of bond lengths can be observed in Figure 3. Progressing from the reactants to the products, it can be seen that the bond lengths of the double bonds in the reactants (i.e. C3-C4, C5-C6, C1-C2) increased from 1.33Å to approximately 1.54Å indicating a change from C=C(sp2) to C-C(sp3). It can also be seen that the bond length of C4-C5 decreased from 1.47Å to 1.34Å indicating a change from C-C(sp3) to C=C(sp2). The values presented are of close proximity to literature values as presented in Table 1.
| C-C(sp3) bond length | C=C(sp2) bond length | VDW radius of Carbon | |
|---|---|---|---|
| Length(Å) | 1.54 | 1.33 | 1.7 |
At the transition state, bond lengths of C2-C3 and C1-C6 are 2.11Å which are smaller than twice the VDW radius of carbon (3.4Å), indicating that there is an overlap of orbitals now that the atoms are closer; all other bonds have bond lengths of approximately half way between C=C(sp2) and C-C(sp3), indicating presence of partial double bonds and a change of hybridization from sp3 to sp2 for C-C bonds and vice versa for C=C.
Vibrational Analysis

The IRC and the imaginary frequency(-948.11 cm-1) shown in Figure 4 and Figure 5 respectively shows that the reaction undergoes a concerted mechanism. (Please click on GIF to see full animation.) In Figure 4, at the imaginary vibration frequency, the two diene carbons can be seen to approach the butadiene carbons at the same time, indicating synchronous and concerted C-C bond formations.
Exercise 2
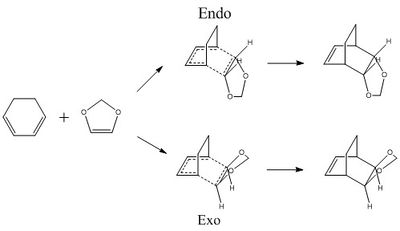
In this exercise, a Diels-Alder reaction between cyclohexadiene and 1,3 dioxole was studied, specifically with regards to inverse electron demand. The reaction was simulated; the reactants and product were optimised using the semi-empirical PM6 basis set, while the endo and exo transition states were optimised using the DFT B3LYP 6-31G(d) method. The reaction scheme is presented in Figure 6.
MO Diagrams
The HOMO-1, HOMO, LUMO and LUMO+1 for the Exo and Endo transition states can be found here and here respectively. The MO diagram of this reaction is presented in Figure 7. In this reaction, it can be seen from the Jmol objects that the HOMO and LUMO of the transition states are both antisymmetric, and by comparing to the MO diagram shown in Figure 7, it can be seen that the transition state orbitals are formed from the LUMO of cyclohexadiene and the HOMO of 1,3 dioxole. This is also confirmed by single point energy calculations for both transition states where the energy of the orbitals were calculated.
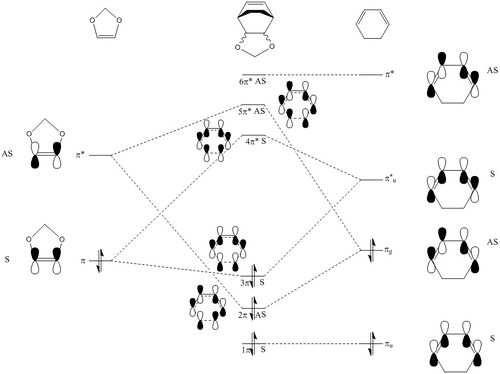
The interaction between the LUMO of the diene and HOMO of the dienophile suggests that this is a reverse electron demand Diels Alder reaction. This can be explained by the presence of oxygens in 1,3 dioxole, which are relatively electron donating, raising 1,3 dioxole's energy levels; the HOMO of the dienophile is then closer in proximity to the LUMO of the diene than its LUMO, as shown in Figure 7, hence the reverse electron demand reaction.
Activation and Reaction Energies
The activation and reaction energies of the reactants, transition states and products for both Exo and Endo pathways are tabulated in Table 2. The energy of reactants were simply calculated by adding up the energy of optimised cyclohexadiene and 1,3 dioxole, assuming that they are separated and have no interactions.
| Endo | Exo | |
|---|---|---|
| Reactants (kJmol-1) | -1313782 | -1313782 |
| Transition states (kJmol-1) | -1313622 | -1313614 |
| Products (kJmol-1) | -1313849 | -1313845 |
| Activation Energy (kJmol-1) | 160 | 168 |
| Reaction Energy (kJmol-1) | -67 | -63 |
00:44, 8 November 2017 (UTC)At least 1 DP compare rhis to chemical reactions you do in snyth labs.
Activation and reactant energies were calculated using the scheme illustrated in Figure 8, the values are tabulated in Table 2. In this Diels Alder reaction, the endo product would be expected to be the kinetic product, due to a stabilised transition state where there are secondary orbital interactions between the oxygens and the diene p orbitals; this is illustrated in the Jmol object for the endo T.S. HOMO in link above. The calculations also agree with the theory since the endo pathway had a lower activation energy. Secondary orbital interactions are not expected or observed in the exo transition state since the oxygen atoms are further away from the diene p orbitals.
The Exo product is expected to be the thermodynamic product as it is sterically less crowded relative to the Endo product. The energy calculations as presented in Table 2 however, does not agree with this theory, since the exo product has a higher energy. This may be explained by steric clashes between bridging and terminal hydrogens as shown in Figure 9. The steric clash in the exo product raising its energy means that the endo product would be both the kinetic and thermodynamic product.

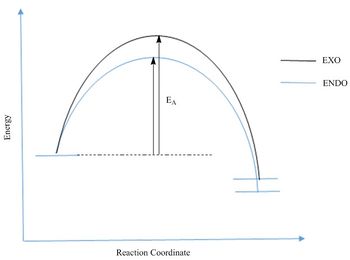
(Please avoid using curved lines like this to represent a reaction coordinate. Here this would imply a non-zero gradient at the reactants and products. It's safer to just draw straight lines as you have below to indicate you are not showing any information about the gradient Tam10 (talk) 11:55, 30 October 2017 (UTC))
Nf710 (talk) 00:48, 8 November 2017 (UTC) This is really well done section ice use of the diagram to show the steric clashes. and how it goes against the normal theory for DA reactions
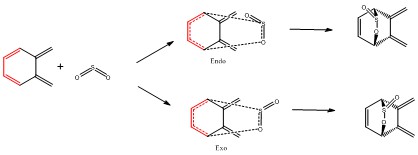
Exercise 3
In this exercise, a hetero Diels Alder reaction between o-Xylylene and SO2 was investigated, where SO2 was used as the dienophile. Both Endo and Exo Diels Alder reactions were simulated as well as the cheletropic reaction. All transition states were optimised using the semi-empirical PM6 method; IRC calculations were also done in both directions from the transition state using the semi-empirical PM6 method. The reaction scheme is shown in Figure 10.
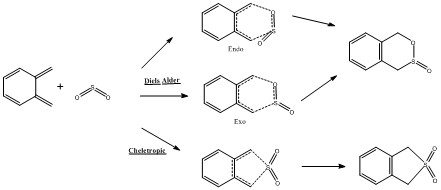
IRC Calculations
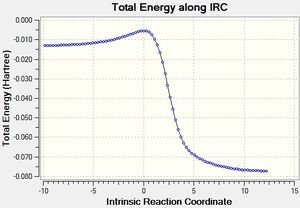
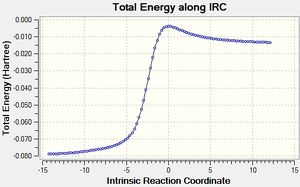
Table 3 below shows the IRC calculations for the Endo, Exo Diels Alder reaction as well as the cheletropic reaction. IRC calculations were done in both directions from the transition states.
| IRC | Total energy along IRC | |
|---|---|---|
| IRC of D-A reaction via endo TS | 
|
|
| IRC of D-A reaction via exo TS | 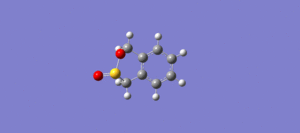
|
|
| IRC of cheletropic reaction | 
|

|
The GIF animations provide an IRC calculation from reactants to products. (Please click on GIF to see full animation.) It can be seen from the energy graph along IRC that the reactions had all converged.
Reaction and activation energies
The reaction and activation energies of the reactions, products and transitions in reactions between o-Xylylene and SO2 are shown in Table 4.
| Endo DA | Exo DA | Chelotropic | |
|---|---|---|---|
| Reactants (kJmol-1) | 154.375263 | 154.375263 | 154.375263 |
| Transition states (kJmol-1) | 237.762673 | 241.745556 | 260.087301 |
| Products (kJmol-1) | 56.956216 | 56.316452 | 0.0133671 |
| Activation Energy (kJmol-1) | 83.387410 | 87.370293 | 105.712038 |
| Reaction Energy (kJmol-1) | -97.41905 | -98.058811 | -154.361896 |
The reaction and activation energies of the reactions are better illustrated in figure 11.

(It would be nice to see the relative energies on this diagram, or delta energies Tam10 (talk) 12:08, 30 October 2017 (UTC))
It can be seen from Table 4 and Figure 11 that the cheletropic product is the most stable, the process is then thermodynamically favoured. This may be due to two strong S=O bonds as well as the aromaticity present. Both the Diels Alder reactions are kinetically favoured due to the lower activation energies. The endo transistion state is again more stable than exo due to secondary orbital interactions between the oxygen and diene p orbitals. Between the two Diels Alder reaction, the exo product is slightly more stable, this may be due to greater steric clashes within the molecule in the endo product.
o-Xylylene is highly unstable, as the molecule does not follow the Huckel Rule and therefore does not benefit from stabilisation gained from aromaticity. From analysing the IRCs of the Diels Alder reactions and chelotropic reaction, the 6-membered ring of o-xylylene aromatises, forming benzene rings during the process, therefore allowing the products to benefit from the stabilisation of the aromaticity in the benzene rings. This is the main driving force for the reactions and explains why the products are thermodynamically more stable than the reactants.
Side reaction between second cis-butadiene fragment in o-xylylene and SO2
A second cis-butadiene present in the six membered ring in o-xylylene can also undergo Diels Alder reaction with SO2. The reaction scheme is shown in Figure 12 below. Components highlighted in red are the cis-butadiene of interest.
The reaction is simulated using the semi-empirical PM6 method, IRC calculations are shown below in Table 5
| Endo TS | Exo TS | |
|---|---|---|
| IRC | 
|
|
The reaction and activation energies of the two Diels Alder reactions are shown in table 6 below.
| Endo TS | Exo TS | |
|---|---|---|
| Reactants (kJmol-1) | 154.375263 | 154.375263 |
| Transition state (kJmol-1) | 923.871512 | 927.121882 |
| Products (kJmol-1) | 172.271342 | 176.715224 |
| Activation Energy (kJmol-1) | 769.496249 | 772.746619 |
| Reaction Energy (kJmol-1) | 17.896079 | 22.339961 |
As can be seen in Table 6, the transition state energies are much higher in both cases compared to when the SO2 approaches the terminal cis-butadiene, making this reaction kinetically very unfavourable. This is as expected, as greater steric clashes is expected when SO2 approaches the internal cis-butadiene, when compared to reaction with the terminal cis-butadiene, this is reflected in the IRC calculations above. The resulting products are higher in energy than the reactants as well, as ring strains and steric clashes are present, making the reaction thermodynamically unfavourable. Therefore, the reaction between the internal cis-butadiene and SO2 is not expected at all, since it is both thermodynamically and kinetically unfavourable.
(The high TS energy is mostly due to the carbon atoms beginning to sp3 hybridise - the large changes in geometry tend to be costly energetically Tam10 (talk) 12:08, 30 October 2017 (UTC))
Second Side reaction
It should also be noted that an electrocyclic reaction is also possible as a side reaction. This process would also be thermodynamically favourable, as it also involves aromatisation of the six-membered ring, hence a competitive reaction against the Diels Alder and cheletropic reactions. The reaction scheme is shown below in figure 13.
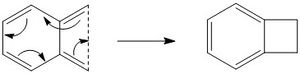
(While thermodynamically favourable, would this be in competition kinetically? As a 4n reaction, this is hard to achieve thermally. Have a look at the HOMO for o-xylylene: it would have to undergo conrotation to reach the TS. As it turns out, this is significantly more costly than all other reactions. You might want to consider what would happen also when photons are involved (have a look at the LUMO) Tam10 (talk) 12:08, 30 October 2017 (UTC))
Conclusion
A typical Diels Alder reaction, an inverse electron demand and a hetero diels alder reaction were investigated, where Gaussview was employed to optimise the reactants, products and transition states. Frequency analysis and IRC calculations were also carried out.
It can be concluded from exercise 1(typical Diels Alder reaction) that only molecular orbitals of the same symmetry would interact, giving a non-zero overlap integral, while opposite symmetry molecular orbitals would give an overlap integral of zero.
From exercise 2 (inverse electron demand Diels Alder reaction), it can be concluded that an inverse electron demand is possible when the dienophile contains electron donating groups.The endo pathway was calculated to be kinetically favourable due to presence of secondary orbital interaction in the transition state. Exo transition states are normally thermodynamically favourable due to less steric hindrance, however steric clashes within the molecule in this case meant that the endo product was also the thermodynamic product
Exercise 3 once again showed that the endo pathway is kinetically favoured while the exo pathway is thermodynamically favoured amongst the two, confirming the theory above. The cheletropic product however is the most thermodynamically stable product. A further investigation into a Diels Alder reaction involving the internal cis-butadiene in o-xylylene showed that the reaction is both kinetically and thermodynamically unfavoured and therefore does not pose as a competing reaction.
Reference
- ↑ # L.Pauling and L. O. Brockway, Journal of the American Chemical Society, 1937, Volume 59, Issue 7, pp. 1223-1236, DOI: 10.1021/ja01286a021, http://pubs.acs.org/doi/abs/10.1021/ja01286a021
