MRD:T01574856
Molecular Reaction Dynamics Lab
Exercise 1: H + H2 System
Question 1
On a potential energy surface diagram, how is the transition state mathematically defined? How can the transition state be identified, and how can it be distinguished from a local minimum of the potential energy surface?
The transition state is mathematically defined as the maximum on the minimum energy path which links the reactants and products together. The transition state can be identified on a potential energy surface diagram by looking at the gradient of the potential, at the point of the transition state, the gradient would be zero (dV(ri)/dri=0). This point is distinguished from a local minimum of the potential energy surface by starting trajectories near the transition state and observing the atoms rolling towards the reactants or the products.
What does the second derivative tell us with respect to minima or maxima? The partial derivative in each direction of the surface is what is important here. Rs6817 (talk) 15:26, 28 May 2020 (BST)
Question 2
Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” plot for a relevant trajectory.
The best estimate for the transition state position is at rts=0 and p=0. This is shown below in the contour diagram as this is in the saddle point; the maxima in one direction and the minima in another. This estimate is also supported by the internuclear distances vs time graph below as it shows the distances of the B-C bond and between the atoms A and C moving symmetrically at the same time. No product is formed at this point.
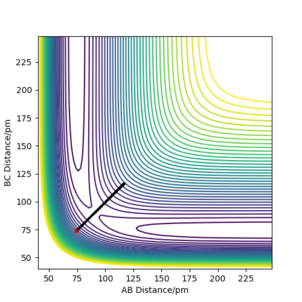
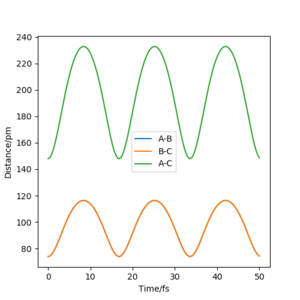
Question 3
Comment on how the mep and the trajectory you just calculated differ.

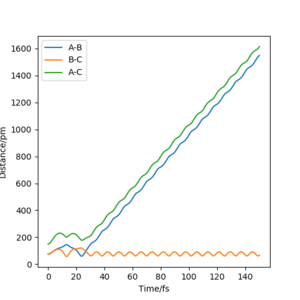
Whilst these graphs show an MEP and a dynamics trajectory - there is no comment supplied here so I am unsure if you undertand the differences between these calculation tpyes. Rs6817 (talk) 15:30, 28 May 2020 (BST)
Question 4
Complete the table above by adding the total energy, whether the trajectory is reactive or unreactive, and provide a plot of the trajectory and a small description for what happens along the trajectory. What can you conclude from the table?
This table tells us that every reaction here had the activation energy required for the reaction to take place but 2/5 times the reaction did not occur. There are other factors such as momenta involved which can effect the outcome.
What do these trajectories tell us about the transition states? What occurs in parts 4 and 5 of this table? The conclusion requires a little more detail. Rs6817 (talk) 15:31, 28 May 2020 (BST)
Question 5
Given the results you have obtained, how will Transition State Theory predictions for reaction rate values compare with experimental values?
Transition state theory uses the properties of the reactants and the transition state to rationalise and calculate the rate of chemical reactions. There are a few assumptions made in transition state theory; one is that all collisions with the required kinetic energy (activation energy) will result in a reaction. Another is that, once the collision occurs and the trajectory passes the barrier, it cannot turn back into the reactants. This does not always occur experimentally as seen in the table above as there are reactants which have the required activation energy which do not react and also there is an example of a collision occuring which resulted in the reactants reforming instead of products forming. In conclusion, this leads to transition theory over-estimating the reaction rates.
Ok good answer however a reference may have helped explore TST a bit more. What does TST say about quantum tunnelling? How does this affect the rate? Rs6817 (talk) 15:33, 28 May 2020 (BST)
Exercise 2: F-H-H System
Question 1
By inspecting the potential energy surfaces, classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved?
To find out if these reactions are exothermic or endothermic a combination of Polanyi's rules and the Hammond's postulate was used. Polanyi's rules look at the early and late barriers of a reaction, this is where the transition state lies on the potential energy surface. An early barrier lies with the transition state closer to the reactants while in a late barrier the transition state lies closer to the products. Translational energy is more efficient to complete the reaction where there is an early barrier but vibrational energy is more efficient in a late barrier reaction. Alongside Polanyi's rules, the Hammond's postulate shows that an exothermic reaction has a transition state resembles the reactants (early barrier) and an endothermic reaction has a transition state resembling the products (a late barrier).
A figure of the PES is required here Rs6817 (talk) 15:36, 28 May 2020 (BST)
Looking at the potential energy surface of the reaction, F + H2, it can be seen that there is an early barrier suggesting a transition state close to the reactants and an exothermic reaction. On the other hand , the reaction of H + HF shows a late transition state, late barrier and therfore an endothermic reaction.
This relates to bond strength as more energy is needed to break the H-F bond than the H-H bond in the exothermic reaction resulting in showing that a H-F bond is stronger than a H-H bond.
Good answer here. Rs6817 (talk) 15:36, 28 May 2020 (BST)
Question 2
Locate the approximate position of the transition state.
The location state of both these reactions would have the same coordinates but in the opposite form. To locate the transition state Hammond's postulate was used alongside looking at the PES graph of the reaction of F with H2. It was known that there was an early transition state so the distance of AB (F-H) would be longer than the already known distance of H2. The point of the transition state was found to produce a single point on the graph at an AB distance of 181.4 pm and a BC distance of 74 pm.
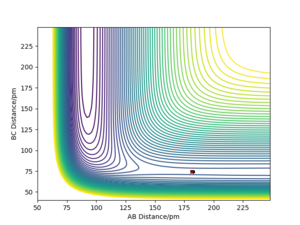
How did you determine this when the H-H-H value was incorrect? Rs6817 (talk) 15:36, 28 May 2020 (BST)
Question 3
Report the activation energy for both reactions.
A resonable estimate was formed by performing an mep with 1500 steps and 0.1 step size from a structure neighbouring the transition state.
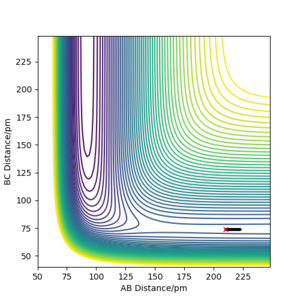
The activation energy fro F + H2 reaction was found by using an AB distance closer to the reactants (210 pm) due to its early transition state giving an activation energy of -0.341 kJ/ mol-1.
The same activation energy value was found for the H + HF reaction but instead due to the late transition state, a BC value of 210 pm was used.
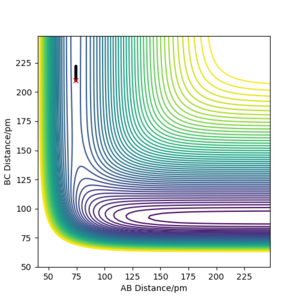
THe PES may not be the most suitable graph to show this. Perhaps an Energy vs time would have allowed you to calculate your values The question asked you to nudge the trajectory just away from your TS which you determined in the above question. Rs6817 (talk) 15:30, 28 May 2020 (BST)
Question 4
In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. Explain how this could be confirmed experimentally.
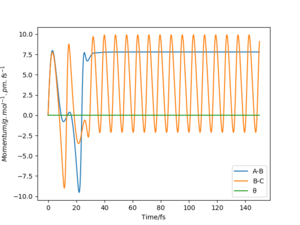
There are two mechanisms of the release of reaction energy; the release of translational kinetic energy and also the release of vibrational kinetic energy. A bomb calorimeter can not differentiate between these 2 mechanisms as it only measures the heat released; therefore IR could be used to measure the radiation emitted from the vibrational energy known as chemiluminescence.The momenta vs time graph below is formed by having an A-B distance of 190 pm and B-C distance of 74 pm with a momentum of -3 and -11 g.mol-1.pm.fs-1 respectively. It shows H-H moving with vibrational energy towards F creating some vibrational energy in H-F. After the collision occurs, H-F now has vibrational energy while the H moves with no vibrational energy.

Chemiluminescence is not possible if the energy is lost as heat. Chemiluniescence is the release of generally visible light from a chemical reaction. Some more detail would help explore the techniques here. How is the energy dissipated after the reaction? Rs6817 (talk) 15:30, 28 May 2020 (BST)
Question 5
Discuss how the distribution of energy between different modes (translation and vibration) affect the efficiency of the reaction, and how this is influenced by the position of the transition state.
Referring back to Polanyi's rule where translational energy is more efficient with an early transition state and vibrational energy being more efficient with a late transition state; the efficiency of the reaction is influenced by which energy is used and where the transition state lies.
This answer does not relate to the F-H-H system, although you did describe Polanyis rules in the previous question. The programme would have allowed you to explore the contribution of vibrational and translational enrrgies depending on exo / endo thermiciity. This would then allow you to determine whether Polanyis rules apply.Rs6817 (talk) 15:30, 28 May 2020 (BST)





