MRD:SanzianaFoia01118973-2018
Exercise 1: H+H2 system
Distinguishing between transition state and minimum points
At a transition structure, both of the components of the gradient of the potential energy surface have values of zero (both slopes are zero). This is also the case with minimum points. Hence, only looking at the gradient (first derivative) would not give enough information to distinguish between transition states and minima.
Therefore, to distinguish between minima and transition structures we can look at the curvature (second derivative) of the potential energy surface orthogonal components. At the transition state, the curvature (second derivative) of one component is negative and the curvature of the other component is positive (in other words, a transition state point, i.e. saddle point, has a local maximum in one direction and a local minimum in another direction).
In contrast, the curvature (second derivative) for a minimum is positive in both components of the potential energy surface.
This part is good--Sw2711 (talk) 14:11, 31 May 2018 (BST)
Estimating transition state position
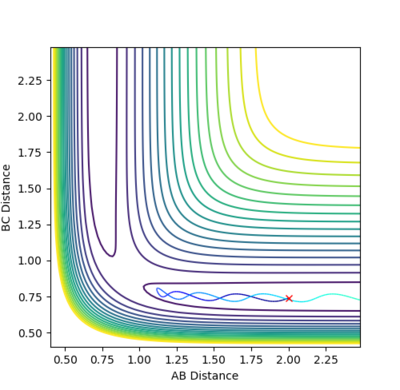
For a symmetric system the transition state occurs at r1= r2=rts. The best estimate of the transition state position for the H+H2 system was found at rts = 0.908 Å. At this position, no oscillations were observed in on the internuclear distance vs time plot, suggesting that the system was at equilibrium and hence confirming that the position corresponded to the transition state. Moreover, the plot shows that A-B and B-C bond distances are not changing over time at pAB=pBC=0, therefore confirming that the state observed, i.e. transition state, is at equilibrium.
Overall, your explanation is good. Just one minor point, `equilibrium` is not a precise word here. `equilibrium` is normally used to describe a reaction in statistical thermodynamics. (as in, if there are millions molecules undergoing the same reaction, overall, the millions molecules have reached an `equilibrium` going forward or backward reaction.) Here, there are only three atoms. So you just need to say, the system has reached the saddle point. --Sw2711 (talk) 14:17, 31 May 2018 (BST)
I think you have missed one question here, talking about the MEP and Dynamics--Sw2711 (talk) 14:18, 31 May 2018 (BST)
Reactive and unreactive trajectories
| p1 | p2 | Total Energy/kcal mol-1 | Reactive? | Description | Contour Plot |
|---|---|---|---|---|---|
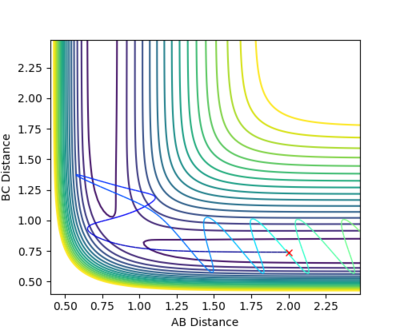
| -1.25 | -2.5 | -99.018 | YES | The reaction path goes all the way from the reactants' channel to the products' channel. No vibrations are observed in the reactants but oscillations can be seen in the products.
Interesting observation. But 'No vibrations are observed' is a very big statement. Is it realistic? --Sw2711 (talk) 14:24, 31 May 2018 (BST) |
|
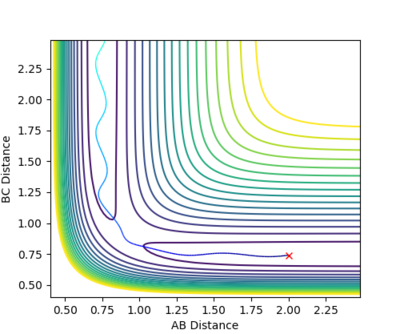
| -1.5 | -2.0 | -100.456 | NO | The reaction path begins in the reactants' channel, approaches (but does not reach) the transition state and also ends in the reactants' channel as the activation energy of the reaction is not reached. The wavy line suggests that the initial molecule is oscillating. | |
| -1.5 | -2.5 | -98.956 | YES | The trajectory is reactive (similar to first trajectory). Molecule vibrates both in the reactants and in the products' channel. | |
| -2.5 | -5.0 | -84.956 | NO | The path starts in the reactants' channel and nearly reaches the transition state, however as it does not overcome the transition state energy, it does not react the products' channel. If you compare the total energy with other reactive cases, you can see this system has clearly acquired enough energy to pass the TS. But why does it not react? This is the question.--Sw2711 (talk) 14:24, 31 May 2018 (BST) | |
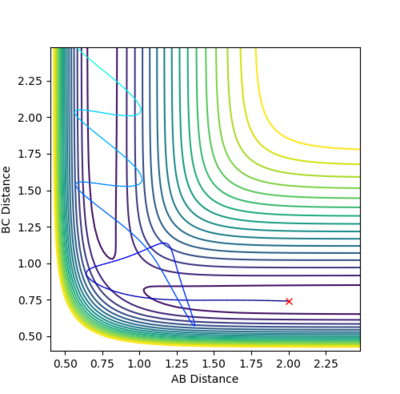
| -2.5 | -5.2 | -83.416 | YES | The trajectory starts in the reactants' channel, the activation energy is reached and the system reaches into the products' channel. |
Assumptions of Transition State Theory
Transition State Theory explains the molecular reaction dynamics by assuming a metastable equilibrium is established between the reactants and the transition state.
- All energies of the reactant atoms are following the Boltzmann distribution - this is only true when systems have enough time to reach a thermal equilibrium.
- Born-Oppenheimer approximation - the nuclei are considered to be much heavier than the electrons, therefore electrons can be negligible, and the particles are assumed to obey classical mechanics. This means that the transition state is only reached if the particles have enough energy,
- Every reaction passes through the the transition state - not always the case at high temperatures.
As I mentioned earlier, 'equilibrium' or 'distribution' is not very applicable here. You are discussing a tri-atomic system only. Furthermore, how does the TST prediction compare to your experimental results above?--Sw2711 (talk) 14:26, 31 May 2018 (BST)
Exercise 2: the F-H-H system
F+H2 and H+HF reactions
F+H2 is exothermic whilst H+HF is endothermic. This can be best illustrated using a surface energy plot. For the first reaction (please see figure below), the reactants are at a higher energy than the products (the products' channel is at a lower energy than that of the reactants), hence the energy change from reactants to products is negative, i.e. exothermic. For the reverse reaction, the the reactants are found at a lower energy hence the reaction is endothermic. This is in accordance with bond strengths (F-H bond is very strong and will readily form, hence the exothermic reaction whilst breaking a F-H bond to form is H-H requires an energy input, therefore the endothermic reaction). Good--Sw2711 (talk) 14:27, 31 May 2018 (BST)
Locating the transition state
The transition state was found at positions rAB=1.811 and rBC=0.744 , where (A, B and C are F, H and H respectively). The energy corresponding to the transition state was found as E=-103.752. I need some more explanation as you did for previous example--Sw2711 (talk) 14:27, 31 May 2018 (BST)
Activating energies for the F+H2 and H+HF reactions
The activation energy was computed using coordinates slightly displaced from the transition state and using the MEP calculation type at 20000 steps so that the last geometry corresponded to the products of each reaction in turn. Using this method, the activation energy for F+H2 was found to be Ea=0.161 whereas the activation energy for H+HF was found as Ea=30.153.
Good, but I need some evidence showing how you actually got these numbers.--Sw2711 (talk) 14:29, 31 May 2018 (BST)
Reaction Dynamics
Reaction conditions for a reactive pathway for the F-H-H system: rAB=0.74, rBC=2.1, pAB=-1.0, pBC=-1.5.
At the start, the H-H molecule vibrates whereas F does not. After the collision, the H-F molecule is formed and oscillates strongly. The energy released in the reaction will be converted from potential energy into kinetic energy. IR spectroscopy could be used for experimental confirmation.
Good. But I need a bit more explanation on how IR could help to determine this. --Sw2711 (talk) 14:31, 31 May 2018 (BST)
I think you have also missed one question about impact of the types of energies to the reaction efficiencies. --Sw2711 (talk) 14:31, 31 May 2018 (BST)