MRD:MaxStocker
Exercise 1: H + H2 system
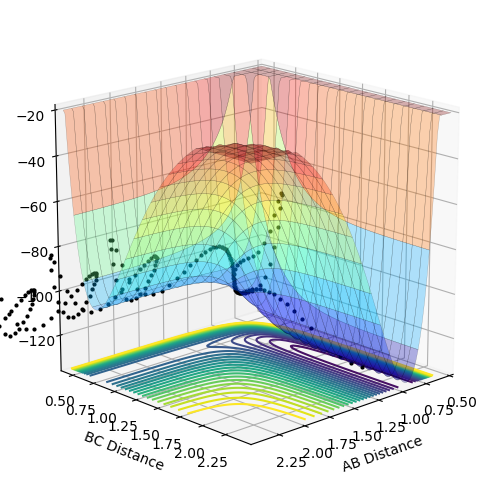
Dynamics from the transition state region
Both gradients are zero for a minimum and a saddle point.
To determine the nature of each stationary point the partial second derivative of each of the two components must be determined.
For a minimum point both components will have second partial derivatives that are greater than zero.
For a saddle point one component second derivative will be greater than zero, while the other will be less than zero.
Good, so how does this relate to your energy surface plot?--Sw2711 (talk) 19:57, 18 May 2018 (BST)
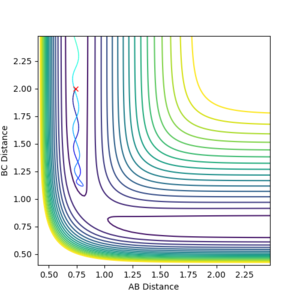
Trajectories from r1 = r2 : locating transition state
An estimate for rts was found to be 0.908 distance units. Looking at the above internuclear separation vs time plot for the distance shows that both the A-B and B-C distances overlap consistently and they is no oscillations, meaning there is no gradient acting on the system, a transition state.
Good--Sw2711 (talk) 19:58, 18 May 2018 (BST)
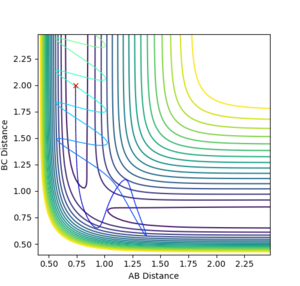
Trajectories from r1 = rts+δ, r2 = rts
The mep line is follows the minimum energy pathway with a straight trajectory, while the dynamic trajectory give a 'wavy' line, this represents the molecule oscillating. The mep seems to purely follow the point of lowest energy and doesn't consider the oscillations Good, but I'd like to see some evidence --Sw2711 (talk) 19:58, 18 May 2018 (BST)
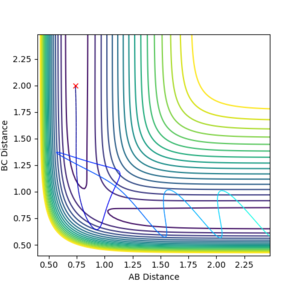
Reactive and Unreactive trajectories
For the table, generally good. But what did you notice about the energy? How does it compare to the overall direction of the reaction?--Sw2711 (talk) 20:03, 18 May 2018 (BST)
One assumption of transition state theory is that the transition state is in quasi equilibrium with the reactants, the dynamic calculation cannot consider an equilibrium.
Transition state theory also assumes that the quantum tunneling effects are negligible, something the calculation also does. If quantum tunneling plays a role in reality we can expect the predicted rate value from TS theory to be slower than reality. For this reaction there is no electron transfer, so we can assume quantum tunneling will have a negligible role here too.
Another assumption is that the reactants approaching the TS are thermally distributed according to the Boltzmann distribution. This has an effect for intermediates that have a lifetime shorter than the time it takes to reach this distribution, as this reaction is 1-step this approximation holds in reality, meaning predictions based on this assumption should come close to reality.
I think the term ‘equilibrium’ is not very applicable here. ‘equilibrium’ is mainly used in statistical thermodynamics. So like…thinking about a reaction A+B<=>C. Statistically speaking there is 80% molecules in this system reacts from the left to the right and 20% the other way around. (about obviously you should think about more like a Boltzmann distribution ). This reaction has reached an equilibrium. But in terms of your system, there are only 3 atoms. So, it is either one way or the other.--Sw2711 (talk) 20:03, 18 May 2018 (BST)
The final assumption is that the TS, once converted to products, will not recross the barrier, this is seen in the calculation, and can be assumed to happen in reality, meaning the predicted rate from TS theory based on this assumption will be faster.
Is this seen in the calculation? What happened in case 4 and 5 then?--Sw2711 (talk) 20:03, 18 May 2018 (BST)
Exercise 2: F-H-H system
PES inspection
F + H2 -> HF + H
Good--Sw2711 (talk) 20:04, 18 May 2018 (BST) Initial conditions
| Atoms | initial distance (Å) | initial momentum (kgms-1) |
|---|---|---|
| F - H | 2.3 | -2.5 |
| H - H | 0.74 | 0 |
Looking at the contour plot of this reaction is can be concluded that the reaction is exothermic, as the product potential energy surface is lower in energy in than the reactants. This means the bond formed (H-F) is stronger than the bond broken (H-H).
HF + H -> F + H2
Good--Sw2711 (talk) 20:04, 18 May 2018 (BST)
| Atoms | initial distance (Å) | initial momentum (kgms-1) |
|---|---|---|
| F - H | 0.92 | 0 |
| H - H | 2 | -8 |
The contour plot shows the reaction is endothermic, the reactants are at a lower energy than the reactants, this is also displayed by the greater amount of momentum needed for the reaction to proceed. As above, this shows the H-F bond is stronger than the H-H bond.
Transistion state approximation
Good--Sw2711 (talk) 20:04, 18 May 2018 (BST)
Using Hammond's postulate we can assume the transition state has character very similar to the reactants of the F + H2 -> HF + F reaction, the reaction is exothermic with a low activation energy. This means for the TS the separation of H-H should be close to the bond length (0.74 Å) while the H-F separation should be larger than the H-F bond length.
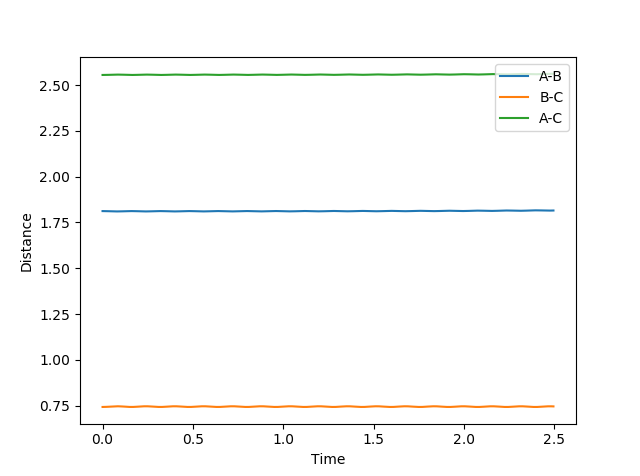
An approximation for the transition state was found to be:
rHF = 1.812
rHF = 0.743
A separation/time graph shows that the distances are not oscillating, meaning the arrangement is balanced in energy and so is the transition state.
Activation energy
Good--Sw2711 (talk) 20:05, 18 May 2018 (BST)
Energy of TS = -103.751 kcal/mol
Energy of HF = H = -133.760 kcal/mol
To find the energy of the HF + H state, 0.1 Å was subtracted to the TS HF distance and an MEP was run, this trajectory ran into the HF + F region of the potential, the final energy of the calculation was taken as the HF + F energy:
| Energy vs Time for MEP of TS to FH + H | Contour plot for MEP of TS to FH + H |
|---|---|
 |

|
Energy of H2 + F = -103.797
To find the energy of the H2 + F state, 0.1 Å was added to the TS HF distance and an MEP was run, this trajectory ran into the H2 + F of the potential, the final energy of the calculation was taken as the H2 + F energy:
| Energy vs Time for MEP of TS to H2 + F | Contour plot for MEP of TS to H2 + F |
|---|---|
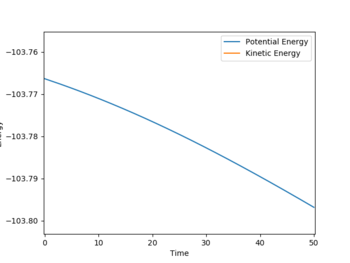 |

|
Activation energy of a reaction is the difference in energy between the products and the transition state, so it can be found for each reaction by finding the difference in energy between the states.
F + H2 -> FH + H
Activation Energy = 0.046 kcal/mol
FH + H -> F + H2
Activation Energy = 30.009 kcal/mol
Reaction Dynamics
For the F + H2 -> FH + H reaction the conditions form before were used:
| Atoms | initial distance (Å) | initial momentum (kgms-1) |
|---|---|---|
| F - H | 2.3 | -2.5 |
| H - H | 0.74 | 0 |
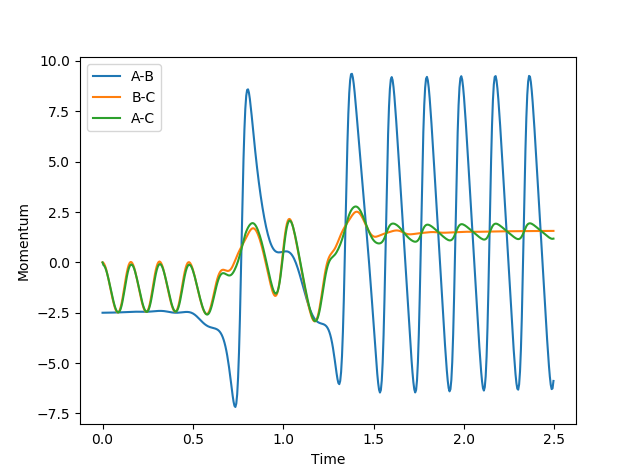
To give the following momentum v time graph:
This shows the reacting H2 H-H bond is initially vibrating (the B-C line is oscillating), the H2 then collides into the F atom, briefly forming the F-H bond, the barrier is recrossed, eventually falling onto the product side. The F-H bond is now formed and virbating (the A-B line is oscillating) while the H-H bond is no more (the B-C line is not oscillating).
Because the reaction is exothermic, energy is produced, however it must be conserved. The excess energy produced in seen in the vibrational energy of the F-H bond. The energy gained from forming a new bond is used vibrationally.
This can be seen using infrared spectroscopy to monitor the vibrational F-H band over time.
Good, but I need bit more explanation on how and why the infrared spectroscopy can monitor the change of energy--Sw2711 (talk) 20:33, 18 May 2018 (BST)
Energy distribution between different modes
Polanyi's Rules
Polanyi's rules state that vibrational energy is more efficient in promoting a reaction with a late transition state (endothermic) than translational energy, the opposite is true for late transition state reactions (exothermic), where translational energy is more efficient in promoting the reaction than vibrational.
Good, although I think Polanyi's rule only mentioned about late TS or early TS. Whether it correlates to endothermic or exothermic, that's Hammond's postulate--Sw2711 (talk) 20:09, 18 May 2018 (BST)
Early Transition states
For the F + H2 reaction the transition state is early, meaning it relies on translational energy this can be seen with relatively low vibrational energy in H2 (low momentum between H atoms) and a higher translational energy (momentum between F and H), the reaction proceeds effortlessly.
| Atoms | initial distance (Å) | initial momentum (kgms-1) | contour plot |
|---|---|---|---|
| F - H | 2 | -0.5 | 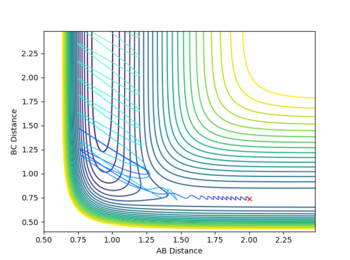
|
| H - H | 0.74 | -0.1 |
If the vibrational energy is raised to a high enough level, it actually inhibits the reaction, the trajectory goes back to the reactants, despite there still being enough translational energy for the reaction to take place. Effectively what happens is the transition state is reached, but there is so much energy the system recrosses the barrier and falls back to the reactants. This can also be seen as the vibrational energy promoting the back reaction which has the late TS.
| Atoms | initial distance (Å) | initial momentum (kgms-1) | contour plot |
|---|---|---|---|
| F - H | 2 | -0.5 | 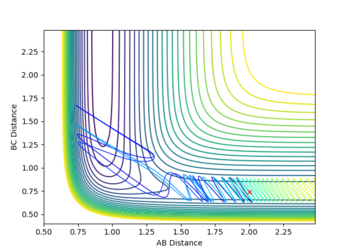
|
| H - H | 0.74 | -2.39 |
Early Transition states
The HF + H reaction has a late transition state, this means that vibrational energy is needed to reach the transition state. This can be seen with the case below, at low vibrational energy for the F-H bond, incredibly high translational energy (H - H momentum) doesn't give a reactive trajectory.
| Atoms | initial distance (Å) | initial momentum (kgms-1) | contour plot |
|---|---|---|---|
| F - H | 0.92 | -0.1 | 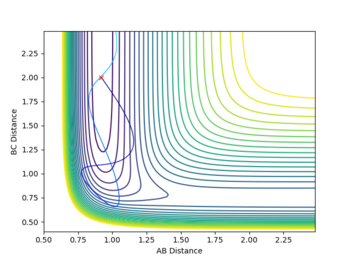
|
| H - H | 2 | -7 |
When vibrational energy is raised and translational energy is lowered (high F-H momentum and low H-H momentum), a reactive trajectory is achieved, true to Polanyi's rule.
| Atoms | initial distance (Å) | initial momentum (kgms-1) | contour plot |
|---|---|---|---|
| F - H | 0.92 | -10 | 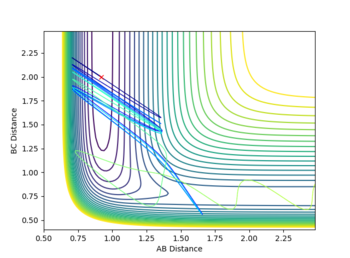
|
| H - H | 2 | -0.1 |
Both arguments are good. --Sw2711 (talk) 20:10, 18 May 2018 (BST)