MRD:LaraMetcalf
Molecular Reaction Dynamics Lab
EXERCISE 1: H + H2 system
A potential energy surface (PES) is a mathematical function which describes the electronic potential energy of a system as a function of the relative atomic positions in space and is widely used as a tool to carry out theoretical studies on molecular reaction dynamics [1].
Dynamics from the transition state region
The total gradient of the potential energy surface at a minimum and transition structure is equal to zero. The distinction between the two structures comes from the sign corresponding to their rate of change of gradient. The potential energy second derivative is positive for a minima and negative for a transition state [2]. Which one? Along which direction? Je714 (talk) 17:36, 31 May 2017 (BST)
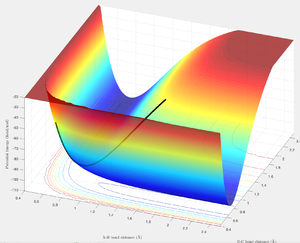
The transition structure linking the two minima is a maximum along the minima energy reaction pathway and thus is called a saddle point [3]. This can be expressed mathematically by Taylor's Theorem [4]:
- If fxxfyy − fx2y < 0 at (a, b) then (a, b) is a saddle point.
- If fxx >0 and fyy > 0 at (a,b) then (a,b) is a minimum point.
At the TS one of the partial 2nd derivatives will be >0 along one of the variables, and <0 along all the rest. Je714 (talk) 17:36, 31 May 2017 (BST)
Trajectories from r1 = r2: locating the transition state
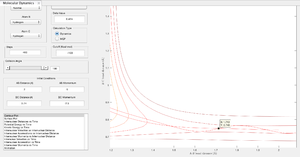
The best estimate of the transition state position rts is found to be equal to 0.90775 Å. The detection of the transition structure can be observed in an internuclear distances vs time plot. The transition state corresponds to where the gradient of the potential energy surface is zero, so it is expected that at rts the H atoms will be stationary - their internuclear separations will remain constant over time (fig 1). At a distance of 0.90775 Å the trajectory disappears (fig 2) confirming this to be a good approximation of the transition state position. Varying the interatomic distance away from the transition state position results in a trajectory that rolls back toward the reactants/products and thus a changing internuclear distance over time is observed (fig 3). Really? That's not what figure 3 is showing. Je714 (talk) 17:37, 31 May 2017 (BST)
Minimum Energy Path (MEP) and Dynamic Calculations
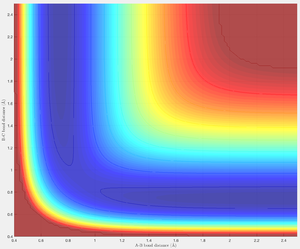
Both calculations produce trajectories that rolls from the reactants H1-H2 H3 in the direction of increasing r1 towards H1 H2-H3 (r1 = A-B) Upon energy minimisation the trajectory converges to the MEP [5]. The MEP calculation generates the minimum energy trajectory thus is restricted to only one degree of freedom, the interatomic positions r1 and r2. The exchange between kinetic and potential energy (due to vibrations) does not occur as a result of the minimisation, and so the generated trajectory does not have an oscillative nature - the kinetic energy is fixed at zero. On the contrary the dynamics calculation does not involve such limitations allowing it to have more degrees of freedom generating an oscillating trajectory, signifying influence of a non-zero kinetic energy.
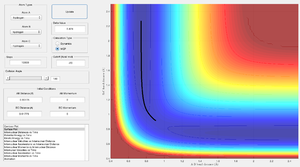
At large t (t): (3 decimal places)
- r1(1.485)= 5.247 Å
- r2(1.485) = 0.739 Å
- p1(1.45) = 2.481
- p2(1.45) = 1.260
Reverting the initial conditions so that r1 = rts + 0.01 and r2 = rts then the reaction would have a trajectory that rolls from H1 H2-H3 towards H1-H2 H3 in the direction of increasing r2.
If the initial positions and final positions were swapped then the corresponding values for final momenta will have the opposite sign.
Reactive and unreactive trajectories
A series of initial condition were tested to determine whether or not they produce a reactive trajectory that starts from the reactants HA-HB and dissociated atom HC to the molecular product HB-HC with dissociated atom HA. Initial momenta p1 of atom HC approaching HB before the transition state and final momenta p2 of atom HA moving away from the molecular product HB-HC
| p1 | p2 | Reactivity |
|---|---|---|
| -1.25 | -2.5 | Reactive |
| -1.5 | -2.0 | Unreactive |
| -1.5 | -2.5 | Reactive |
| -2.5 | -5 | Unreactive |
| -2.5 | -5.2 | Reactive |
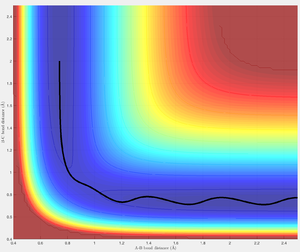
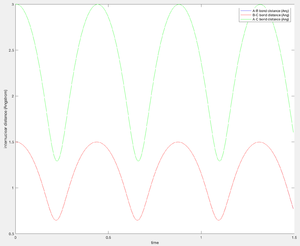
The first set of conditions generates a linear trajectory path from HA-HB HC to the transition structure at approximately 0.908 Å. Before the collision r1 remains constant and r2 steadily decreases as H3 approaches H2. There is sufficient energy to overcome the activation energy barrier allowing the trajectory to roll towards the products. After the transition structure r1 steadily increases as HA moves away from HB from the molecule formed HB-HC, the oscillating trajectory signifies the vibrational energy of the HB-HC bond.
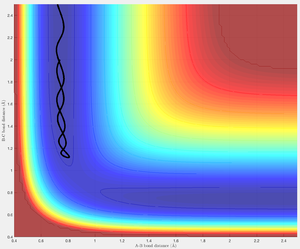
Increasing p1 and p2 (initial momenta), relative to the first set of conditions, results in an unreactive trajectory. Before the transition structure r1 has a slight oscillation (vibrational energy of A-B) and r2 steadily decreases as HC approaches HB. At r1=0.810 Å and r2=1.120 Å the trajectory bounces back toward the reactants. This suggests amount of energy available to the system is not sufficient to overcome the activation energy barrier - the reaction is unsuccessful. It is evident that the vibrational energy of HA-HB is retained upon the return of the system to the reactants.
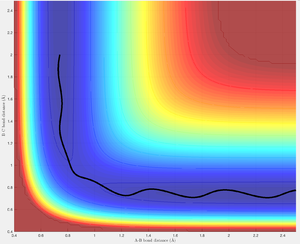
Increasing p2 from -2.0 to -2.5 provides the energy required to overcome the barrier thus allowing the reaction to proceed. The trajectory rolls from the reactants to the products passing through the transition structure at 0.893 Å. Again the vibrational energy of the HA-HB then HB-HC bonds are observed before and after passing through the transition structure respectively.
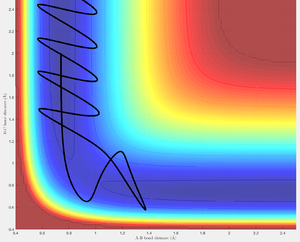
With the fourth set of initial momenta conditions the trajectory rolls from the reactants and passes through the transition structure illustrating an initial formation of the HB-HC bond. Soon after crossing the transition region the trajectory returns to the reactants - this is an example barrier re-crossing. The trajectory bounces several times within a small region about transition state position before returning back to the reactants.
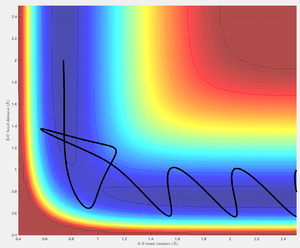
The trajectory approaches the transition structure along the direction of decreasing r2 at a constant r1. Again the trajectory displays barrier re-crossing before eventually bouncing across the barrier toward the product HB-HC and dissociated atom HA.
The final two sets of initial momenta have relatively large p2 values. Both systems have significant translational kinetic energy as H atom A moves away from the formed molecule HB-HC. At high kinetic translational energies of atoms/molecules it becomes increasingly difficult to justify the differences in the reactivity of the generated trajectories. This suggests the application of Transition State Theory is unsuitable for dynamic molecular systems of this type - this method is limiting.
Transition State Theory
The transition state is the known as the 'critical configuration' that corresponds to the highest point on a minimum energy reaction pathway on a potential energy surface [6]. If the system is able to reach the critical geometry then a successful reaction is highly probable. The transition structure is a stationary point thus is identified as the saddle point, with associated reaction coordinates, on a potential energy surface. The transition state theory is a cumulative a series of assumptions used to approximate the equilibrium rate constants for thermally driven molecular reactions [7]. The TST stemmed from thermodynamic, kinetic-theory and statistical mechanic treatments [7].
Development of the Transition State Theory
The thermodynamic treatment stems from the Van't Hoff equation [7] which relates the change in the equilibrium constant (keq) to a given change in temperature (T) for a given enthalpy change (delta H):
- .
The kinetic-theory [7] explains the temperature dependence of reaction rates as a function of collision frequency and the statistical mechanical treatment focuses on equilibrium reaction rates in terms of the motion of molecules and statistical distribution of molecular speeds [7] - initially modelled by Maxwell and Boltzmann.
Main Features and Assumptions of the Transition State Theory [7]
- (1) Rates can be calculated by focusing on the "activated complex" - what happens before the transition state is reached are not important if one rate dominates the overall rate of reaction.
- (2) The activated complex is formed in 'quasi-equilibrium' i.e. the complex is in equilibrium with the reactants and products passing through - the concentrations of reactants and products can be determined by changing the concentration of one species e.g. removing the products.
- (3) Motion of the system is treated as 'free translational motion' - expressed by kinetic-theory.
Using the Transition State Theory to Determine Reactivity
The TST ignores the dynamical recrossings that can lead back to the reactants (also known as tunneling) so this calculation tends to overestimate the rates within the classical mechanic framework [7]. For a successful reaction the molecular system must have sufficient energy to overcome the activation energy barrier and thus generate a reactive trajectory. The Arrhenius equation relates the activate energy to the reaction rate for a particular reaction scheme, k=Ae(-Ea/RT) [8]. The relationship shows how a small activation energy barrier will correspond to a larger reaction rate constant - the reaction will go faster. It is likely that the experimental activation energy barrier will be larger than the calculated value thus TST will likely overestimate the rate relative the the experimental rate for a given reaction.
Good job looking up information. Just a small point -- the statistical mechanical treatment does not really hold here, since we are not looking at an ensemble of particles, just at a simple triatomic collision in isolation. The biggest point to be made here, was the recrossing limitation, and how that overestimates the rates when compared to experiment. Because that's the most relevant limitation that you observe in this lab. Je714 (talk) 17:40, 31 May 2017 (BST)
EXERCISE 2: F - H - H system
Reaction Energetics
The F + H2 reaction is exothermic. An exothermic reaction is defined as the reaction path from a higher (reactants) to lower total potential energy (products) [9]. The trajectory shows the approach of F to H2 from infinite separation (as it is dissociated): as A-B separation decreases the potential energy decreases to a minimum indicating the H-F bond formation and H-H bond dissociation is an exothermic process.
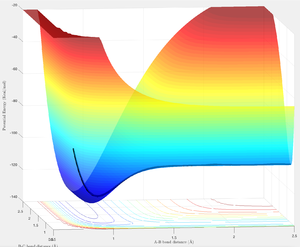
By definition the reverse of an exothermic reaction is endothermic [9], this is indicated by the trajectory generate by the H-F + H reaction. H-F bond breaking and H-H bond forming is an endothermic and enthalpically unfavourable process. The reactants have a lower total potential energy than the products.
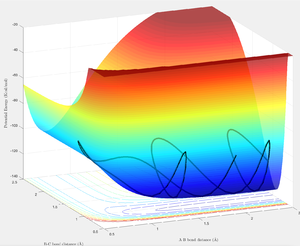
The H-F bond formation has a more negative (exothermic) reaction enthalpy indicating H-F to have a stronger bond than H2.
Location of Transition State and Determination of the Activation Energies
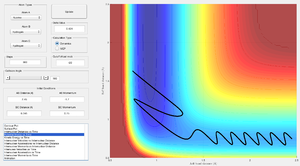
Rates of reactions are controlled by the free energy of the transition state and Hammond postulated that the structure of a given transition state will more closely resemble that of the reactants or products depending on which is closer in energy [10]. He concluded that for an exothermic reaction the transition state will be more structurally similar to the reactants i.e. the interconversion between the reactants and transition state will involve only a small reorganization of molecular structure. And conversely for an endothermic reaction the transition state will be more structurally related to the products. The transition structure is expected to be closer in energy and structure to the reactants - longer A-B and shorter B-C distance so point of intersection further right in the saddle region was selected to determine the location (see figure 12) and energy of the transition state.
Approximate location of the transition state (3 dp):
- A-B (F-H) = 1.713 Å
- B-C (H-H) = 0.748 Å
Activation energies (2 dp):
- Energy of the reactants (F + H2) = -103.80 kcal/mol
- Energy of the products (F-H + H) = -134.00 kcal/mol
- Energy of the transition structure = -96.47 kcal/mol
- Activation energy for formation of HF + H = +7.33 kcal/mol (=-96.47--103.80)
- Activation energy for formation of F + H2 = +37.53 kcal/mol (=-96.47--134.00)
How did you get the Eas? They're not very accurate Je714 (talk) 17:41, 31 May 2017 (BST)
Reaction Dynamics
Mechanism of Release of Reactant Energy
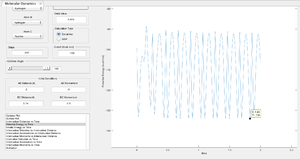
The amplitude of the oscillating trajectory reflects the magnitude of the defined initial momenta values. The reactants produce an oscillation of smaller amplitude than the products as p1 (H-H) < p2 (F-H). The trajectories before and after the transition state however differ by the period of the generated oscillations - this indicates differences in the translational kinetic energy in the reactants and products. It has been discussed previously that the molecular product H-F has a stronger bond than H-H so it is expected to ‘see’ a greater translational kinetic energy in the products than the reactants. This is displayed by the trajectory, the oscillation period for the product H-F (A-B) is larger than that of the reactant H-H (B-C). As a result of the conservation of energy, the total energy of the system must remain constant [11]. Therefore the product state should have a lower potential energy than the reactants to balance the total energy (kinetic and potential) of the system so that it remains constant, suggesting the reaction to be exothermic - earlier concluded to be the case as for the reaction for F + H2. This could be confirmed by different spectroscopic methods such as calorimetry and IR/Raman spectroscopy.
- Heat-flow calorimetry is commonly used to thermally monitor reactions and thus determine whether a reaction is exothermic or endothermic. The heat transfer under e.g. constant pressure is monitored as the reaction proceeds and consequently the enthalpy change can be determined. Thermodynamically ΔH=qp at constant pressure (where qp is the heat flow at constant pressure), otherwise known as Hess's Law [12]. Exothermic reactions release heat thus if exothermic the calculated value for the enthalpy change will be negative in sign (visa versa for endothermic reactions).
- IR/Raman spectroscopy is used to determine the vibrational states of molecules/matter. If the reactants possess less translational kinetic energy and thus vibrational energy then the IR spectra for the reactants should display lower intensity peaks relative to the peaks in the IR spectra of the products.
Good experimental suggestions. Je714 (talk) 17:41, 31 May 2017 (BST)
Polanyi's empirical rules
Polanyi's empirical rules focus on the importance of the transition state location in enhancing reactivity for activated reactions [13]. This model has enabled the prediction of the relative efficacy of vibrational and translational excitations in promoting different molecular dynamic reactions.
- Polanyi's rules [13] state that vibrational energy is more efficient at promoting late transition states (endothermic reactions according to Hammond Postulate [10]) than translational energy. And conversely translational kinetic energy is more efficient at promoting 'early' transition states (exothermic reactions).
The reaction from H + H-F provides an illustration of these rules. This reaction is endothermic, thus is an example of a late-barrier reaction - the transition state is closer to the products in energy and structure according to Hammond's Postulate [10]. According to the Polanyi's Empirical Rules the reactivity should be enhanced more efficiently by high vibrational energy (and low kinetic energy). When initial conditions were chosen so that the reactant H-H had high vibrational energy (high pHH) and low translational kinetic energy (small pHF) a kinetically driven trajectory was generated.
Not all systems obey Polanyi's Rules as a result of their empirical nature (experimentally determined). As the rules are experimentally determined they effectively relate to the average behaviour of a reacting system [14]. The reaction H2 + F is an example where the calculated trajectory deviates from Polanyi's predictions. This reaction is exothermic so according to Polanyi's rules it is expected that high translational kinetic energy would promote the early transition state and thus enhance the reactivity. However for the reactive trajectory to reach completion a higher initial momenta for H-H (pHH) than initial translational kinetic energy (pHF) was required. This may be due to the errors in the computational calculation of the reaction trajectory thus does not accurately portray was would be observed experimentally.
Missing some examples here with your trajectories. Je714 (talk) 17:42, 31 May 2017 (BST)
References:
- ↑ Laganà, Antonio, and Antonio Riganelli. Reaction And Molecular Dynamics. 1st ed. Berlin, Heidelberg: Springer Berlin Heidelberg, 2000. Print..
- ↑ Collett, Charles T, and Christopher D Robson. Handbook Of Computational Chemistry Research. 1st ed. New York: Nova Science Publishers, 2010.
- ↑ Lewars, Errol G. "The Concept Of The Potential Energy Surface". Computational Chemistry (2010): 9-43. Web. 11 May 2017.
- ↑ Jeffrey, Alan. Essentials Of Engineering Mathematics. 1st ed. Boca Raton: Chapman & Hall/CRC Press, 2004. Print.
- ↑ Koistinen, O-P. et al. "Minimum Energy Path Calculations With Gaussian Process Regression". Nanosystems: Physics, Chemistry, Mathematics (2016): 925-935. Web. 20 May
- ↑ Fueno, Takayuki. Transition State: A Theoretical Approach. 1st ed. Tokyo: Gordon and Breach Science Publishers, 1999. Print.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 Laidler, Keith J., and M. Christine King. "Development Of Transition-State Theory". The Journal of Physical Chemistry 87.15 (1983): 2657-2664. Web. 26 May 2017.
- ↑ Kotz, John C, and Paul Treichel. Chemistry & Chemical Reactivity. 1st ed. Fort Worth: Saunders College Pub., 1996. Print.
- ↑ 9.0 9.1 Petrucci, Ralph H. General Chemistry. 1st ed. Toronto, Ont.: Pearson Canada, 2011. Print.
- ↑ 10.0 10.1 10.2 Carey, Francis A, and Richard J Sundberg. Advanced Organic Chemistry. 1st ed. Norwell: Springer, 2007. Print.
- ↑ Khine, Myint Swe, and Issa M Saleh. Models And Modeling. 1st ed. Dordrecht: Springer Science+Business Media B.V, 2011
- ↑ Mikulecky, Peter, Michelle Rose Gilman, and Kate Brutlag. AP Chemistry For Dummies. 1st ed. Hoboken, N.J.: Wiley Pub., 2009. Print.
- ↑ 13.0 13.1 J. Clayden, N. Greeves and S. G. Warren, Organic Chemistry, Oxford University Press, Oxford, 2012
- ↑ Zhang, Zhaojun et al. "Theoretical Study Of The Validity Of The Polanyi Rules For The Late-Barrier Cl + Chd3reaction". The Journal of Physical Chemistry Letters 3.23 (2012): 3416-3419. Web. 26 May 2017.
