MRD:LJK16
Question 1
What value do the different components of the gradient of the potential energy surface have at a minimum and at a transition structure? Briefly explain how minima and transition structures can be distinguished using the curvature of the potential energy surface.
The transition state is a saddle point on a potential energy surface. The transition state is a transient state with the maximum energy, in a reaction coordinate. The gradient, the first differential (dV/dr), has a value of zero at the minimum and maximum (transition state) stationary points of the potential energy surfaces. The second differential term (dV^2/dr^2) has a value greater than zero for the minimum point and less than zero for the maximum point. By taking the second differential the minima and transition structures can be distinguished.
Question 2
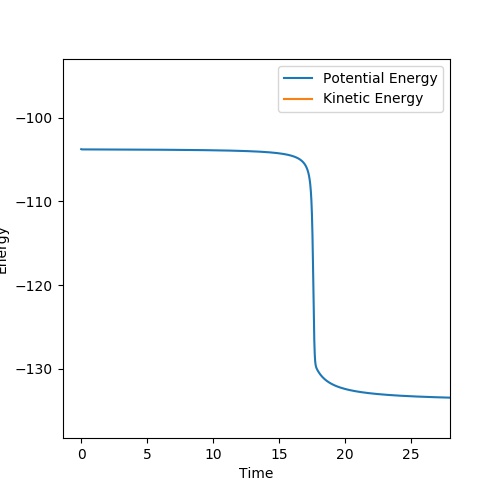
Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” plot for a relevant trajectory.
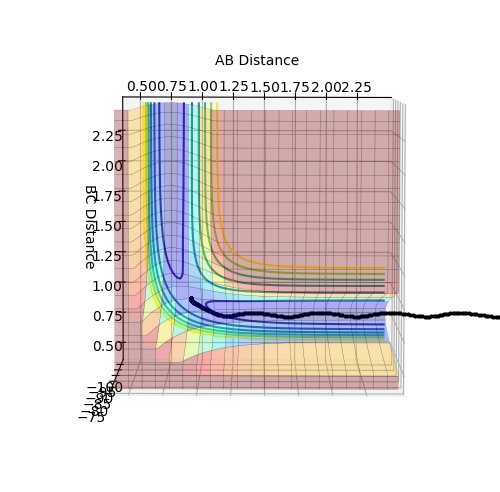
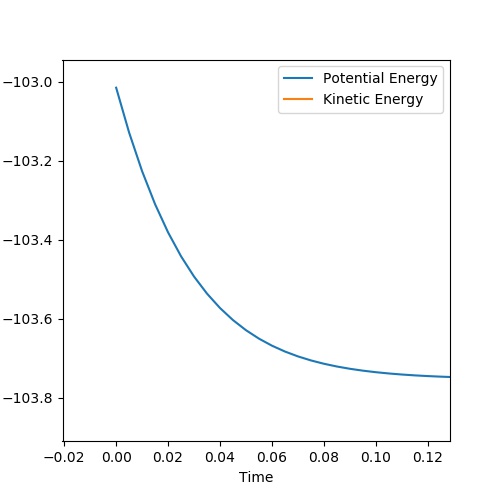
The transition state position is at rts = 0.908 Angstroms. Force acting on the hydrogen molecule is given by the negative of the derivative of the potential energy surface with respect to the coordinate.[1] Since the gradient at the transition state is zero, force will be zero. Thus the kinetic energy is zero and so all energy is potential here. As can be seen in the Internuclear Distances vs Time plot below there is no oscillation which indicates that this is a transition state as there is no kinetic energy.
The bond distance is equal between the three H atoms at the transition state because this is a symmetrical system.
Mm10114 (talk) 17:04, 25 May 2018 (BST) Nicely explained and supported with a relevant plot.
Question 3
Comment on how the mep and the trajectory you just calculated differ.
The minimum energy path, mep, is a trajectory with infinitely slow motion. For the trajectory surface plot viewed in the dynamic calculation, the reaction path starts at the transition state and moves completely to the products: the reaction forms products. The dynamic trajectory oscillates indicating vibration of H-H. For the mep calculation, the reaction path starts at the transition state and starts to move towards the products but does not go to completion and so products are not formed. The mep trajectory does not oscillate and so is not vibrating. For the mep the velocity is equal to zero at any point in time whereas the trajectory has varying velocity.
Mm10114 (talk) 17:07, 25 May 2018 (BST) These are all good observation. However, you do not explain why there is oscillation in dynamics and not in MEP. Why MEP does not go to completion and dynamics do? Some more in-depth explanation is necessary here.
Question 4
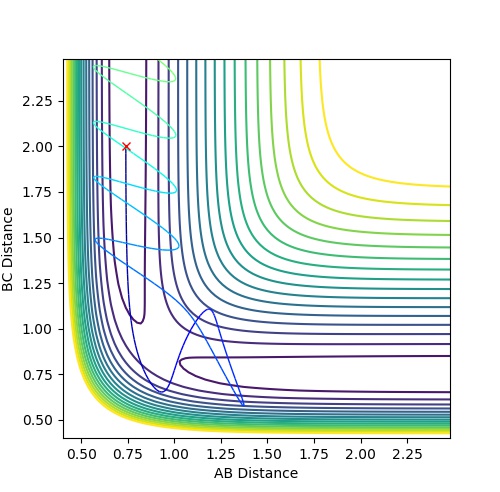
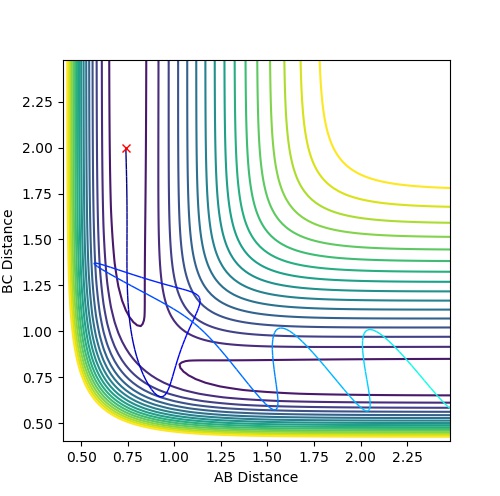
Complete the table by adding a column with the total energy, and another column reporting if the trajectory is reactive or unreactive. For each set of initial conditions, provide a plot of the trajectory and a small description for what happens along the trajectory.
| Image | p1 | p2 | total energy | reactive or unreactive | Description |
|---|---|---|---|---|---|
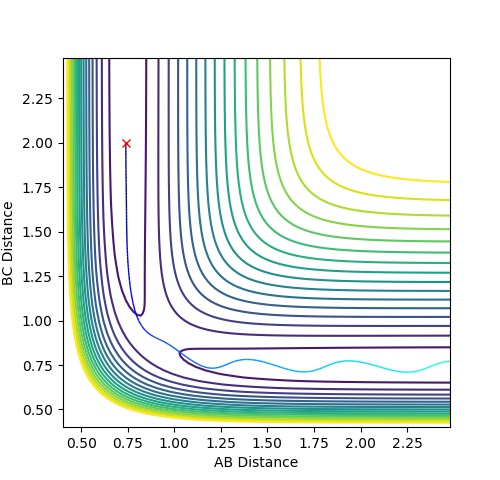
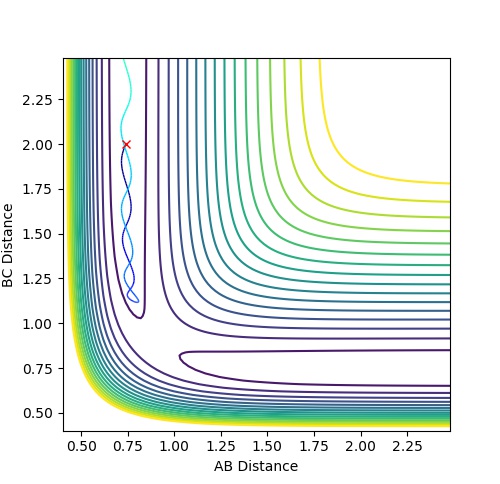
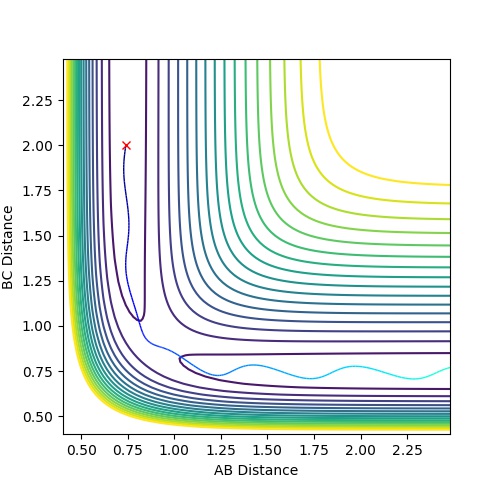
| 1 | -1.25 | -2.5 | -99.018 | reactive | The reactants proceed to the products via the transition state without oscillation before but then oscillates after the TS (corresponding to vibration). This implies that
the H atom collides with the hydrogen molecule, BC, whilst it is stationary. But the hydrogen molecule, AB, then vibrates. |
| 2 | -1.5 | -2.0 | -100.456 | unreactive | The system approaches the transition state but does not cross this region and remains as reactants. Atom A moves towards vibrating BC but the energy is not great
enough to overcome the activation barrier to reach the transition state. |
| 3 | -1.5 | -2.5 | -98.956 | reactive | The reactants proceed to the products via the transition state. The trajectory oscillates before and after the transition state corresponding to vibration of the BC and AB
molecules. |
| 4 | -2.5 | -5.0 | -84.956 | unreactive | The system crosses the transition state region and a bond forms in the product but then the system reverts back to the reactants in a process of barrier recrossing. A
approaches BC not oscillating and forms A-B-C. But BC reforms now oscillating and so the system does not reach the products and so the trajectory is unreactive. |
| 5 | -2.5 | -5.2 | -83.416 | reactive | The system crosses the transition state region and recrossing occurs but overall the system proceeds to the products. A approaches BC not oscillating and the product formed, AB, then oscillates. |
Mm10114 (talk) 17:44, 25 May 2018 (BST) Could you draw some conclusions of your observations here? In case 4 and 5 the momenta are much higher than in 1-3, but only one is reactive. So what does that mean?
Question 5
State what are the main assumptions of Transition State Theory. Given the results you have obtained, how will Transition State Theory predictions for reaction rate values compare with experimental values?
- Molecular systems that have crossed the transition state in the direction of products cannot revert to reactants.
- In the TS, motion along the reaction coordinate may be separated from other motions and treated classically as translation.
- The system passes through the lowest energy transition state on the potential energy surface.
The TS theory overestimates the reaction rate values as it does not take into account that some trajectories revert back to the reactants at the transition state, as shown in the fourth image above. BC and A may collide with enough energy but this does not necessarily mean that products are formed.[2] Recrossing can occur and can either revert to reactants (4) or proceed to products (5).
Question 6
Classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved?
F + H2 is exothermic which means that the products are lower in energy than the reactants. Thus the products are more stable and therefore the bonds in the products are stronger than bonds in the reactants. A stronger H-F bond is formed (bond enthalpy of formation is negative) and a weaker H-H bond is broken (bond enthalpy of dissociation is positive).
H + HF is endothermic which means that the products are higher in energy than the reactants. Thus the products are less stable and therefore bonds in the products are weaker. The H-F bond is the strongest single bond and breaking this to form a weaker bond is an endothermic process since the positive bond enthalpy of dissociation is a greater value than the negative bond enthalpy of dissociation here.
Mm10114 (talk) 17:10, 25 May 2018 (BST) Good.
Question 7
Locate the approximate position of the transition state.
The Hammond postulate states that the transition state resembles the products or reactants. In exothermic reactions the transition state is closer in energy to the reactants. Therefore the transition state for F + H2 resembles the reactants. In endothermic reactions the transition state is closer in energy to the products. As a result, the transition state in H + HF will resemble the products.[1] Therefore for both reactions the H-H bond distance, AB, could be considered and optimised for both systems until finding the transition state.
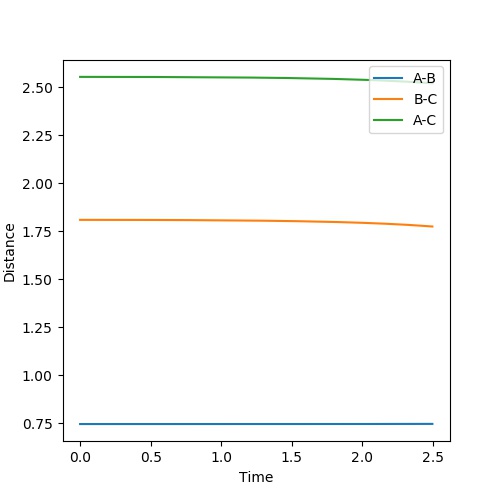
The transition state approximate position is the same for both reactions as the same complex is formed of [H-H-F]. The approximate position is where H-H = 0.7450 Angstroms and H-F = 1.8089 Angstroms.
Mm10114 (talk) 17:12, 25 May 2018 (BST) It seems that after some time, your distances start to shift. What does this tell you about your estimate for the position of transition state?
Question 8
Report the activation energy for both reactions.
Activation energy = transition state energy - reactant energy. The transition state energy was found at - 103.15 Kcal/mol for both reactions as they form the same complex at the transition state.
1) F + H2: -103.15 - (103.75) = 0.60 Kcal/mol
2) H +HF: -103.15 - (132.5) = 29.735 Kcal/mol
Mm10114 (talk) 17:15, 25 May 2018 (BST) Are you sure about the unit? Should it not be kcal/mol and not Kcal/mol? Be careful in the future, as science is not indifferent for upper/lower case in units. Yours could be read as Kelvin * calorie / mol instead of kilocalorie / mol.
Question 9
In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. How could this be confirmed experimentally?
The release of energy corresponds to an exothermic chemical reaction. This occurs when the reactants have higher chemical energy than the products. Stored chemical potential energy is the enthalpy of that specie. Enthalpy changes can be measured experimentally by calorimetry.
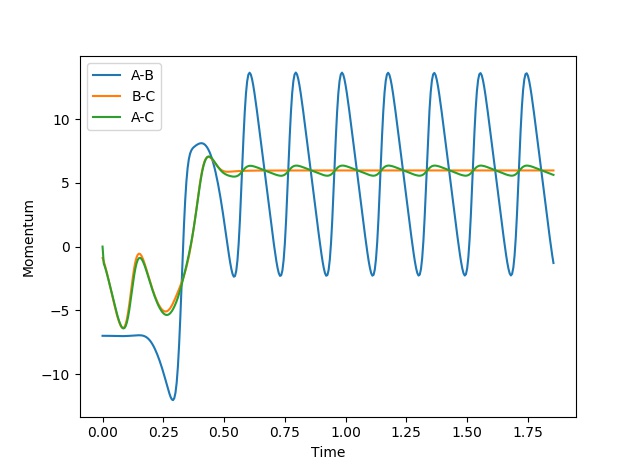
If enthalpy decreases during a chemical reaction, a corresponding amount of energy must be released to the surroundings; this is an exothermic reaction. If the enthalpy increases during a reaction, a corresponding amount of energy must be absorbed from the surroundings; this is an endothermic reaction. This is the law of conservation of energy. Therefore in this reaction it would be expected that there would be a decrease in potential energy for an increase in the kinetic energy. F + H2 is exothermic and so H-F is expected to oscillate more than H-H. The internuclear momenta vs time graph (A = F, B = H and C=H) below confirms this as it shows how AB has greater oscillation than BC.
Question 10
Discuss how the distribution of energy between different modes (translation and vibration) affect the efficiency of the reaction, and how this is influenced by the position of the transition state.
Translational or vibrational energy helps reactants overcome the activation barrier. Polanyi's empirical rules indicate that a vibrational energy is more efficient in overcoming the activation barrier when it is a late transition, and translational energy is more efficient when it is an early transition.[1]
F + H2 is exothermic and so according to Hammond's postulate has an early transition state. Therefore an increase in translational energy over the vibrational energy results in a reactive reaction.
HF + H is endothermic and so has a late transition state and so an increase in vibrational energy over translational energy would result in a reactive reaction.
References