MRD:KGW15
EXERCISE 1: H + H2 system
Q1
At a transition structure, both ∂V(rA-B)/∂rA-B and ∂V(rB-C)/∂rB-C are 0. The same applies to the minima. The two can be distinguished with the second derivatives: at the transition state, ∂V(rA-B)/∂rA-B (where A is the leaving atom) is positive while ∂V(rB-C)/∂rB-C is negative.
(Your derivatives and second derivatives are the same here. The derivative measures the potential for the whole system, not just one coordinate. Finally, the coordinates at the TS are not the same as R_AB and R_BC when determining what the curvature is. If you recall the diagram of the MEP, the reaction coordinate changes direction and becomes 45º - a linear combination of R_AB and R_BC. Tam10 (talk) 16:14, 6 June 2017 (BST))
Q2
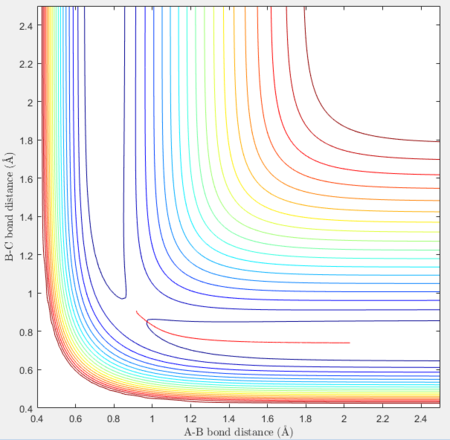
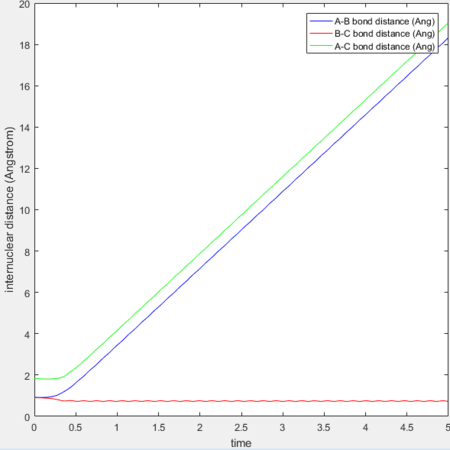
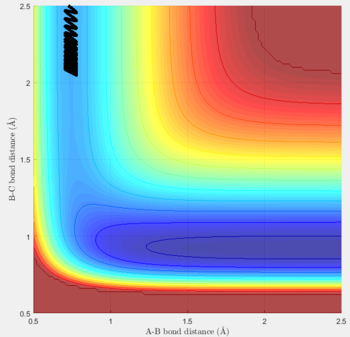
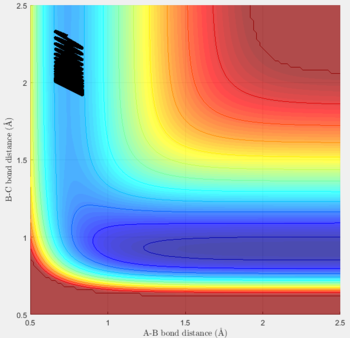
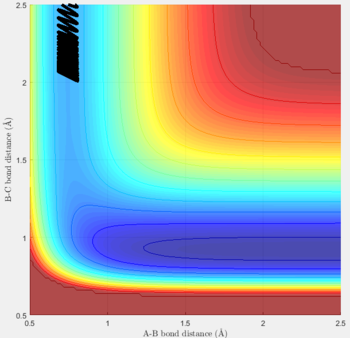
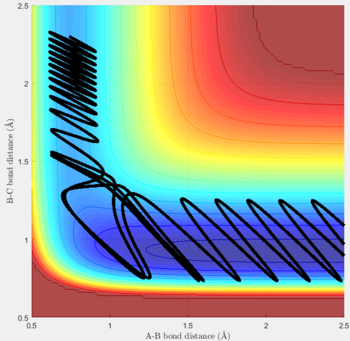
Transition state position (rts): 0.90775Å
It was estimated from the intersection point in the original "bond distances vs time" graph, then refined by trial and error. The constant distance can be implied as the transition state position because with particles with zero momenta sitting at the saddle point would be motionless and stay at that position.

Q3
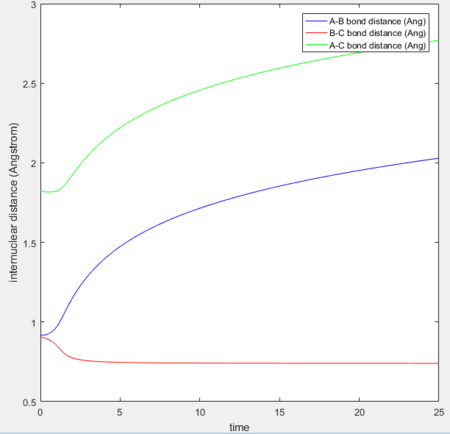
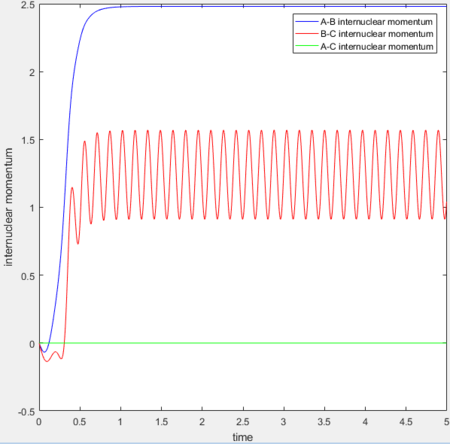
MEP vs 'relistic' trajectory: Molecular vibration is taken into consideration for Dynamics calculation but not in MEP. It is due to the fact that MEP assumes the atoms have zero velocity and thus zero momentum.
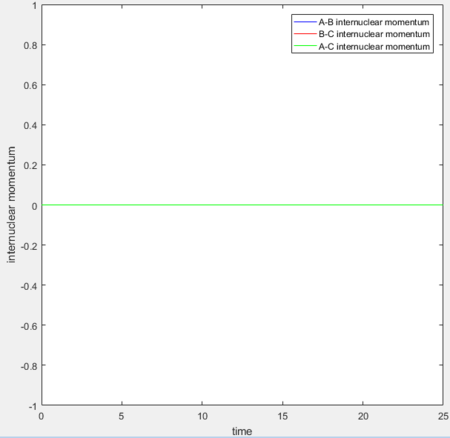
Setting rAB= rts+0.01, rBC = rts and the momenta pAB = pBC = 0,
for MEP: the final value of the position rAB(t) = 2.029Å, rBC(t) = 0.741Å and the average momenta pAB(t) = 0 and pBC(t) = 0 at large t.
for Dynamics: the final value of the position rAB(t) tends to infinity, rBC(t) = 0.740Å and the average momenta pAB(t) = 2.481 and pBC(t) = 1.241 at large t.
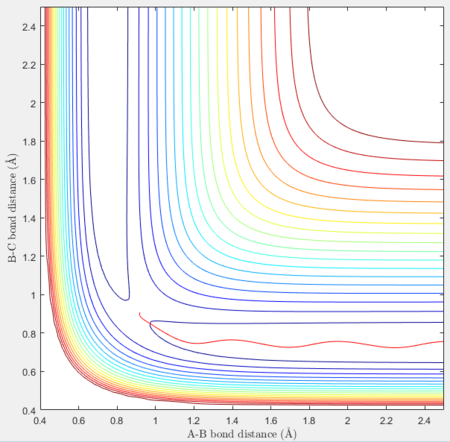
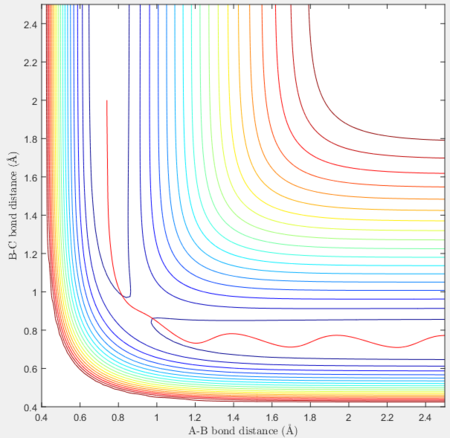
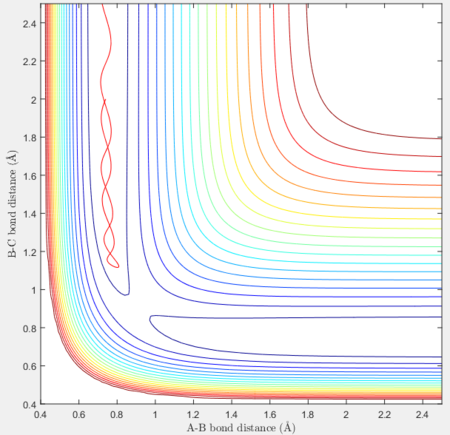
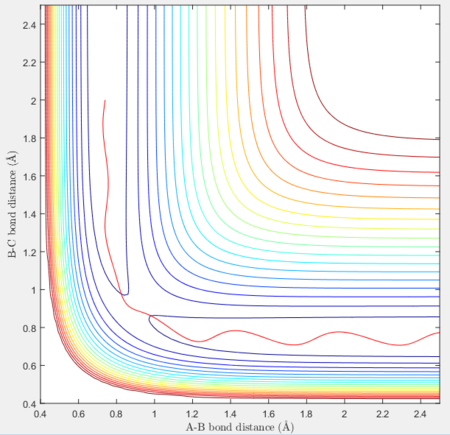
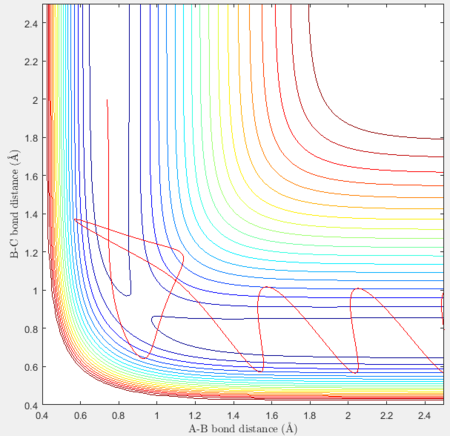
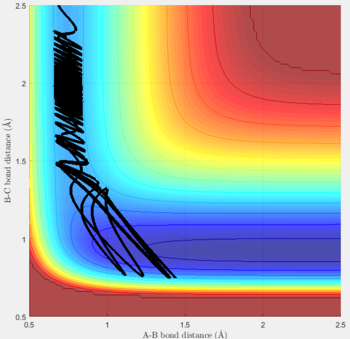
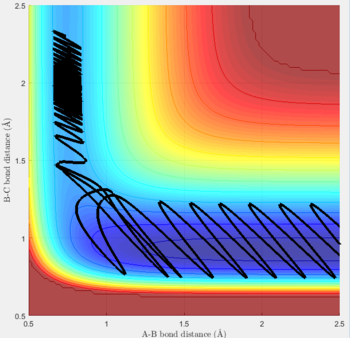
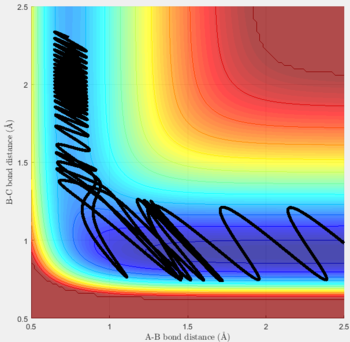
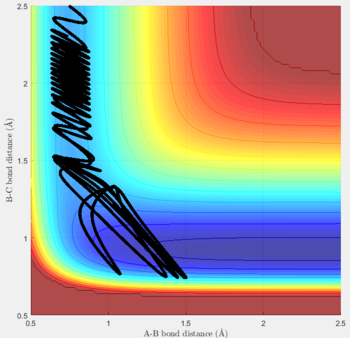
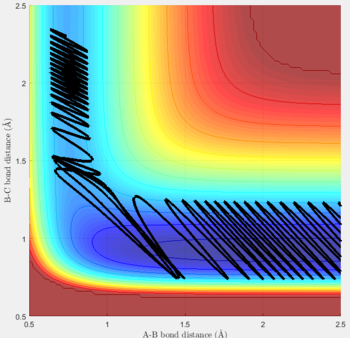
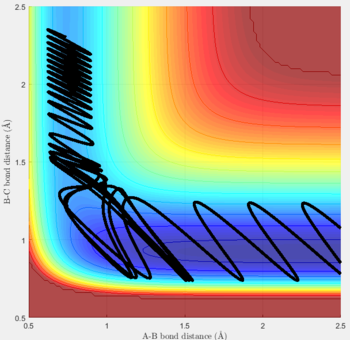
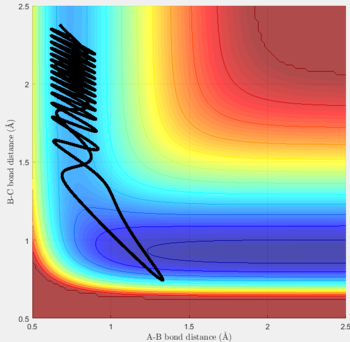
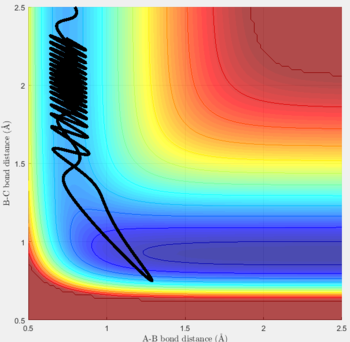
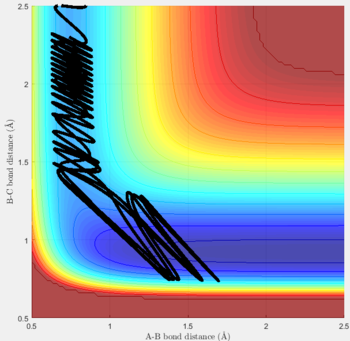
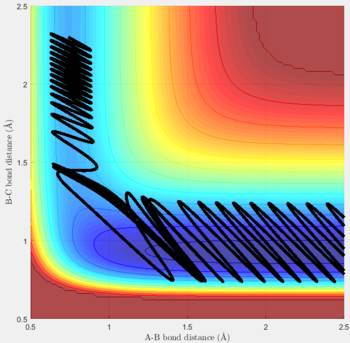
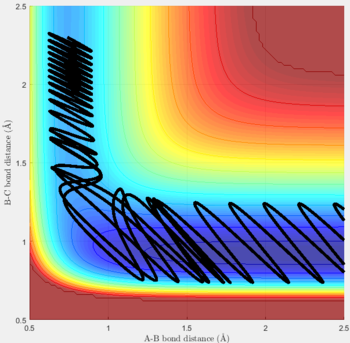
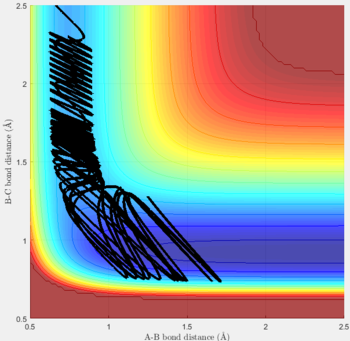
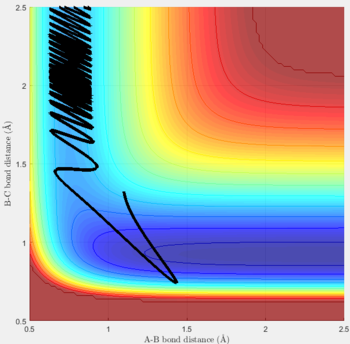
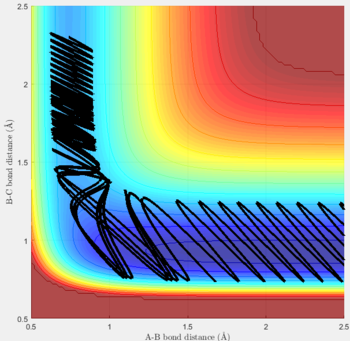
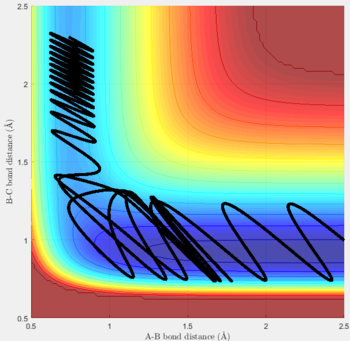
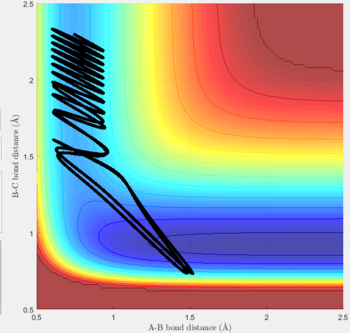
Reactive and unreactive trajectories
For the initial positions r1 = 0.74 and r2= 2.0, trajectories with the following momenta combination were run:
Transition State Theory
Transition state theory (TST) assumes that once the reactants reach the transition state, it will proceed towards the products. From the calculations made above, TST fell short in some cases where it did not predict systems being able to revert to the reactants after reaching transition state. Therefore predicted reaction rate values will be higher than experimental values since it does not take into account the rate of backwards reaction of transition states.
EXERCISE 2: F - H - H system
PES inspection
The F + H2 reaction is exothermic. It can be seen on the PES that H-F has a lower potential energy than H-H. Since H + HF is the reverse of the F + H2 reaction, it is endothermic. From this, we can interpret that H-F bond strength is higher than that of H-H.
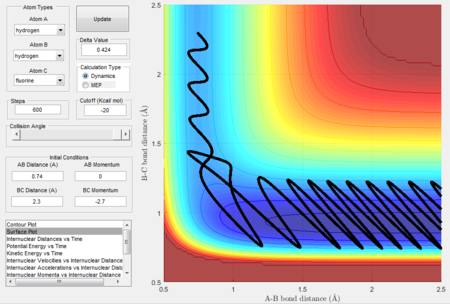
Transition state approximation
Since the system is not symmetric, the H-F and H-H bond length is not the same at the transition state. Moreover, it is difficult to extract information of the transition state from the trajectory calculated above.
An approximation can be made initially by a method similar to bisection: two initial momenta, p1 and p2 are searched such that the fluorine with p1 will not cross the activation barrier, whereas the one with p2 will have the sufficient energy to just cross the barrier. Thus the momentum px with the exact amount of energy to lie on top of the activation barrier will be between (p1, p2). The position of the atom can be found accordingly:
 |
 |
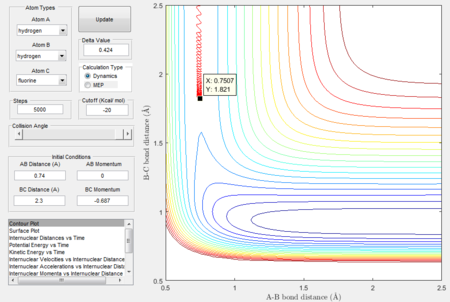
MEP calculations are carried out to verify the approximation:
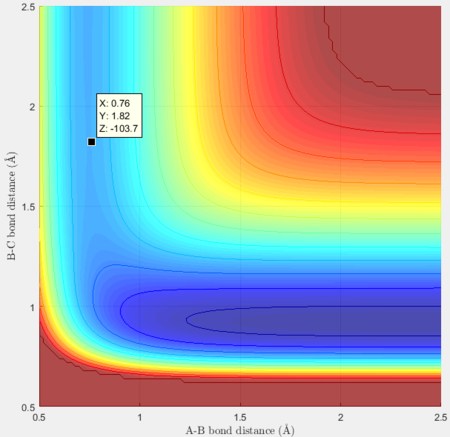
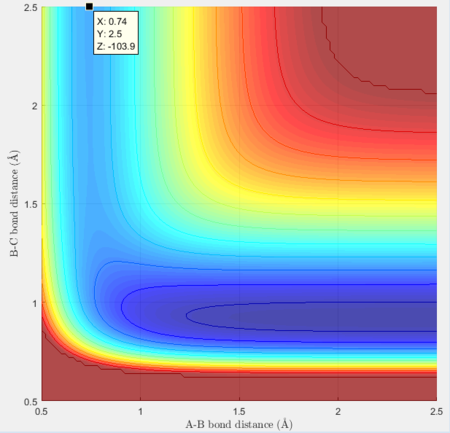
Thus the approximate transition state is at H-F = 1.821Å and H-H = 0.7507Å
Activation energy for both reactions
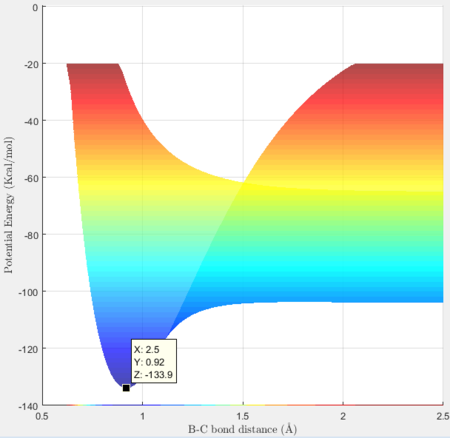
The activation energy for a reaction is the difference between the potential energy of the transition state and that of the reactants.
Ideally, the potential energy for the reactants should be measured where the reactants are separated infinitely. However, due to the limitations of the model, the maximum separation can only be 2.5Å, which potentially introduces a large error.
Activation energy for F + H2= -103.7 + 103.9 = 0.2 kcal/mol, which is largely different to literature value of 1.68 kcal/mol[1]
Activation energy for H + HF = -103.7 + 133.9 = 30.2 kcal/mol
 |
 |
 |
Reaction dynamics
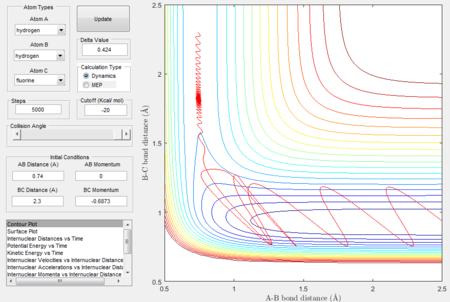
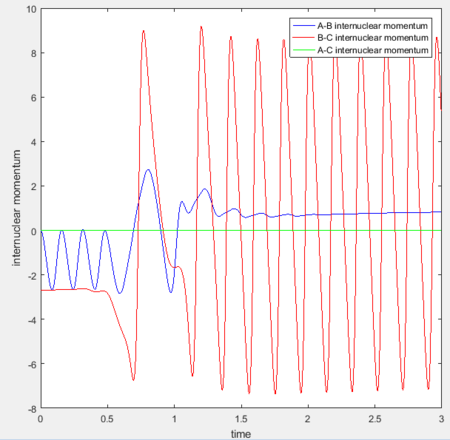
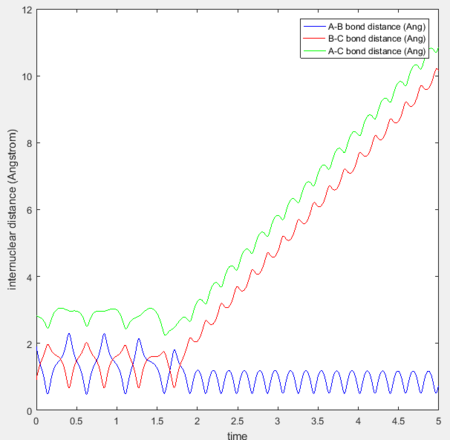
Initial conditions were set as previously shown in Fig 10: H-H distance = 0.74Å, H-F distance = 2.3Å, H-H momentum = 0, H-F momentum = -2.7. Simulation results are as shown:
 |
 |
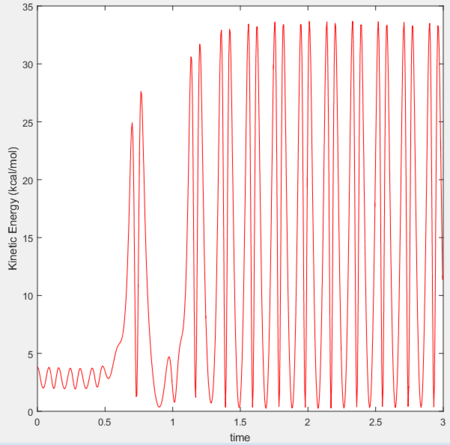 |
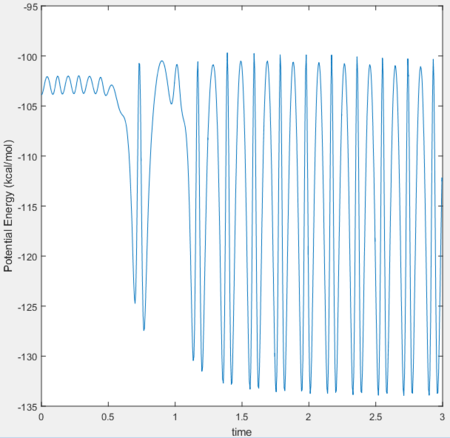
Since it has been established that the reaction is exothermic, the loss in potential energy is believed to have transferred to kinetic energy. To confirm whether conservation of energy is held for the system, the 'PE over time' and 'KE over time graph' are analysed:
| Average before collision | Average after collision | Difference | |
|---|---|---|---|
| PE | -102.9 | -116.48 | -13.58 |
| KE | 2.835 | 16.9705 | +14.1355 |
Potential energy is transferred as kinetic energy in the system.
The momenta of the system before and after the reaction can be analysed qualitatively: as F-H is formed, the amplitude of vibration is larger than that of H-H before the bond breaks. This is expected, as KE = p2/2m. The increased kinetic energy is manifested in the H-F vibration.
Experimentally, the released PE can be measured as an increase in temperature of the reaction system.
Effect of initial momenta on reactivity
F + H2
F + H2 was tested for pHH in the range -3 to 3, with rHH = 0.74, momentum pFH = -0.5.
atom A = H, atom B = H, atom C = F
From the plots, it can be concluded that there exists a discrete range of values of pHH which leads to a reaction. These values lie between each reactive result. It should be noted that shown above was not an exhaustive result, there may be more values in between.
Using the same initial position, another plot was created with pFH = -0.8 and pHH = 0.1:
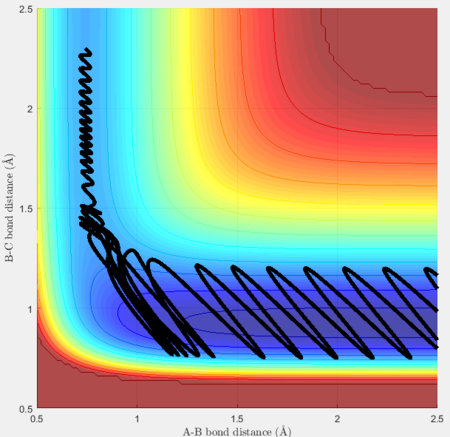
An observation can be made regarding the magnitude of pHH that even when it is smaller, representing a lower vibrational energy, the H-F bond formed still has a high vibrational frequency.
H + HF
An optimisation of momenta was carried out for the reverse reaction with rHH = 1.9Å, rHF = 0.92Å.
atom A = H, atom B = H, atom C = F
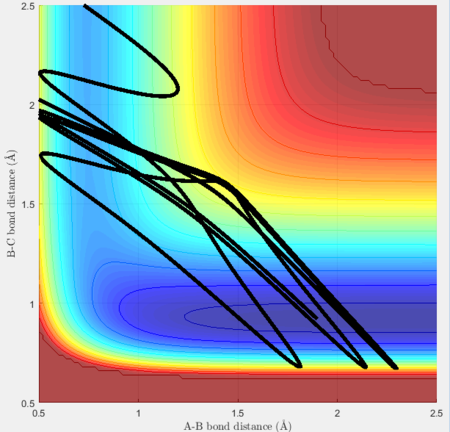 |
 |
Polanyi's empirical rule
Polanyi's empirical rule states that for a reaction with early transition state, energy stored in translation mode is more effective for promoting the reactants through the activation barrier; whereas for a reaction with late transition state, energy stored as vibration mode is more effective for promotion[2]. For F + H2, since it is exothermic, its transition state is early and thus it is favoured for reactions with high transnational energy. As for H + HF, the endothermic reaction has a late TS and vibration mode energy is more effective for its promotion through the barrier. This was demonstrated in Fig 44, where the H-F vibration was maximised while minimising the translational energy of the incoming H.
References
[1]: Jun Chen, Zhigang Sun, and Dong H. Zhang, The Journal of Chemical Physics 142, 024303 (2015)
[2]: J. C. Polanyi and W. H. Wong, The Journal of Chemical Physics 51, 1439 (1969); doi: http://dx.doi.org/10.1063/1.1672194