MRD:JDN15
EXERCISE 1: H + H2 system
Value of gradient at minimum and at transition state.
Q: What value does the total gradient of the potential energy surface have at a minimum and at a transition structure? Briefly explain how minima and transition structures can be distinguished using the curvature of the potential energy surface.
The total gradient of the potential energy surface at minimum = 0.
The total gradient of the potential energy surface at a transition structure = 0.
While their total gradient are both zero, they can be differentiated by observing the curvature of the potential energy surfaces. At the minima, the curvature of the potential energy surface has a positive second derivate (i.e concave). However, at the transition structure, it is a saddle point as the second derivate will be negative.
(As a saddle point it will the maximum along once axis and the minimum along another. Lt912 (talk) 03:22, 2 June 2017 (BST))
Estimating Transition State Position (rts)
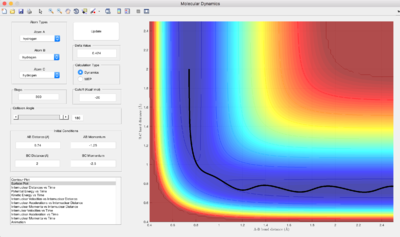
Q: Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” screenshot for a relevant trajectory.
rts = 0.9075 Å

As see in the "Internuclear Distance vs Time" graph, we can observe that there is minimal oscillations. This represents that the atoms are near its equilibrium positions and it is near its minimum potential energy, which is its transition state.
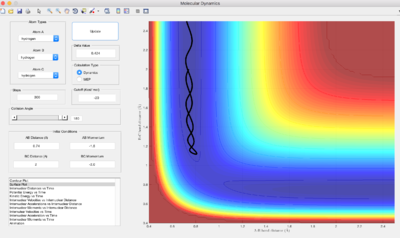
Difference in Trajectory between MEP and Dynamic Calculation methods
Q: Comment on how the mep and the trajectory you just calculated differ.
Using MEP calculation type, the trajectory does not show any oscillations and hence produces a straight line. However, if dynamic calculation type is used, oscillations are observed as waves in the graph. This is because in MEP calculations, the velocity is reset to 0 after each step, therefore the trajectory will move in the direction of the steepest descent each step. This corresponds to the minima of the potential surface, which is the valley floor. In dynamic calculations, the atoms possess an initial velocity that affects its motion and hence trajectory, resulting in oscillations.
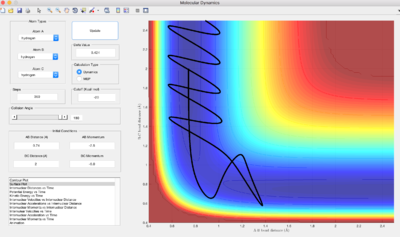
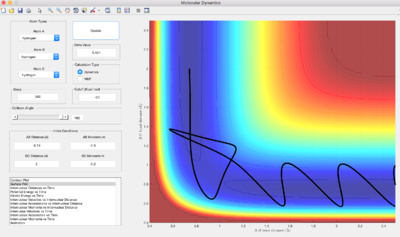
Reactive or Unreactive Trajectories
Q: Complete the table by adding a column reporting if the trajectory is reactive or unreactive. For each set of initial conditions, provide a screenshot of the trajectory and a small description for what happens along the trajectory.
Assumptions of Transition State Theory
Q: State what are the main assumptions of Transition State Theory. Given the results you have obtained, how will Transition State Theory predictions for reaction rate values compare with experimental values?
1. The Transition State Theory assumes that a quasi-equilibrium occurs between reactants and activated transition state complexes. This is when even though the reactant and products are not in equilibrium, the reactant is in equilibrium with the activated transition state complex.
2. The flux of activated complexes in the two directions are independent of each other.
3. Each intermediate is long-lived enough to reach a Boltzmann distribution of energies before continuing to the next step.
4. Unless atoms or molecules collide with enough energy to form the transition structure, the reaction does not occur.
5. The reaction system will pass over the lowest energy saddle point on the potential energy surface.
6. Barrier recrossing is not allowed.
(Based on your dynamics calculations, are these assumptions valid? Which of these are unlikely to be true in a real system? Lt912 (talk) 03:26, 2 June 2017 (BST))
EXERCISE 2: F - H - H system
PES INSPECTION
Q. Classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic).
F+H2 → HF + H (1) is an exothermic process, while HF + H → F+H2 (2) is an endothermic process.
Q. How does this relate to the bond strength of the chemical species involved?
In (1), weaker H-H bonds (432 kJmol-1) are broken and stronger H-F bonds are formed (-565 kJmol-1), resulting in a overall stabilisation (exothermic) of the molecule.
In (2), stronger H-F bonds are broken and weaker H-H bonds are formed, resulting in a destabilisation (endothermic) of the molecule.
Q. Locate the approximate position of the transition state.
The approximate positions are rH-F = 0.181 Å and rH-H = 0.745 Å.
Q. Report the activation energy for both reactions.
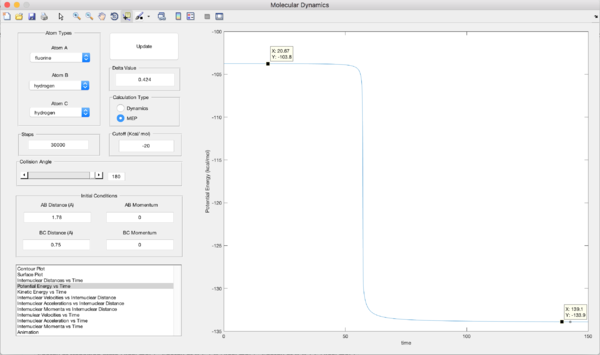
Energy of Transition F-H-H = -103.3 kJmol-1
Energy of F+H2 = -103.8 kJmol-1
Activation of Energy for (1) = -103.8 - 103.3 = 0.6 kJmol-1
Energy of HF + H = -133.9 kJmol-1
Activation Energy for (2) = -133.9 - 103.3 = 30.5 kJmol-1
Reaction Dynamics
Q. In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy.
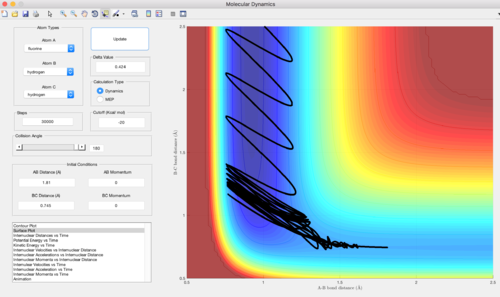

At the start of the reaction, H2 has very little vibrational energy. As F moves towards the H2 , it is observed that the system recrosses into the transition state and back many times. The product HF and H eventually forms. HF possess a large amount of vibrational energy while H has little vibrational energy / translational energy only and moves away from HF.
(It looks like you were starting your trajectory from the transition state rather than from the reactants. I am also not convinces that the transition satte is being recrossed may times. The cyclic motion seems to happening in the product channel.Lt912 (talk) 03:28, 2 June 2017 (BST))
Q. How could this be confirmed experimentally?
This can be confirmed via Infrared (IR) chemiluminescence spectroscopy, which will detect the infrared vibration when the excited molecules return down to ground state.
The experiment is also exothermic and hence a calorimeter can be used to determine if the reaction has occurred.
Q. Discuss how the distribution of energy between different modes (translation and vibration) affect the efficiency of the reaction, and how this is influenced by the position of the transition state.
A reaction can only occur if the reactants possess sufficient energy that is distributed in the correct modes. If the energy modes are not in the correct level, the reaction will not proceed as the molecules will not approach in the right orientation or efficiently.
It is important to consider the energy modes at the transition state as it will affect the efficiency of the reaction. For example, in an exothermic reaction, according the Hammond's postulate, the energy modes of the transition state will resemble more like the reactant. Similarly, for an endothermic reaction, Hammond's postulate suggest that the energy mode of the transition state will resemble more like the product.
(This is not a correct interpretation of Hammond's postulate - which does not address distribution of energy in the transition state. I think there is some confusion with Polanyi's rules here. Lt912 (talk) 03:34, 2 June 2017 (BST))