MRD:Ekart
Exercise 1: H + H2 system
The gradient of the potential energy surface is 0 at both the minimum and transition structure. The difference between them is only in curvature, while at a transition state there is a maximum, meaning that the surface is convex at that point (second derivative is smaller than 0), at an actual energy minimum the energy surface has a minimum, the curve is concave (the second derivative is larger than 0).
(Not entirely true, the transition state is a maximum along one axis, but a miminum along another. Lt912 (talk) 11:50, 12 June 2017 (BST))
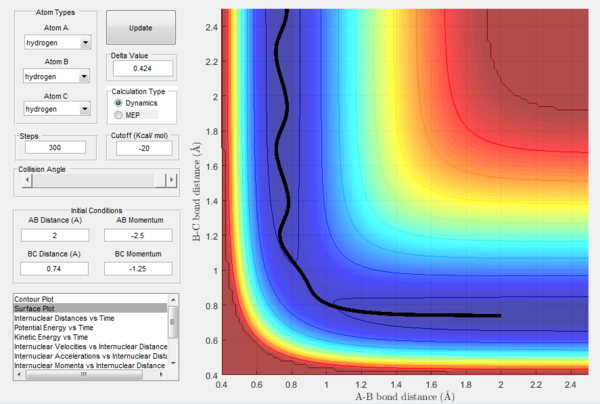
rts
rts was found to be at around 0.76 Å. At that distance the system remained in transition state for about 1.8 seconds, before starting to move towards lower energy level. Although not the actual transition state, it is the closest estimate that could be obtained, as changing the initial distances for as little as 0.001 Å in increments up and down for 0.01 Å resulted in shorter times at (almost) transition state.
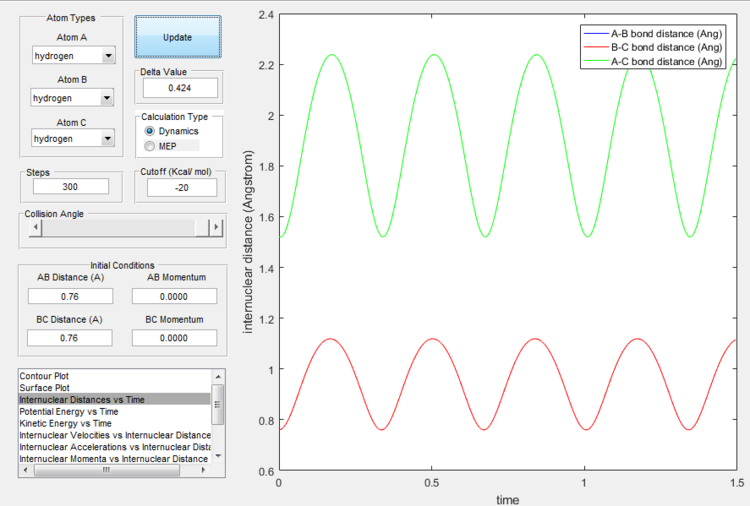

(This is quite far from the transition state, I'm sure you could do better. The lines showing distances with time should be virtually straight. Lt912 (talk) 11:52, 12 June 2017 (BST))
Dynamics vs MEP
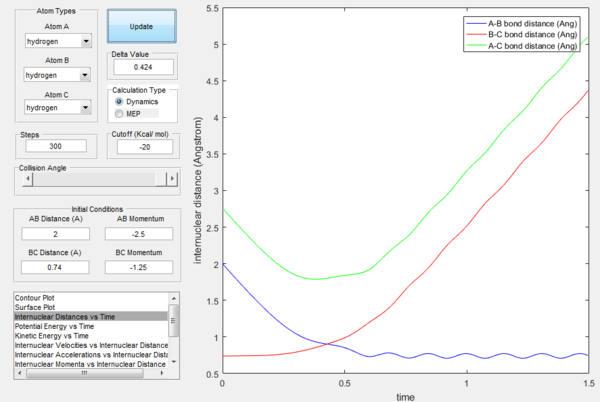
Dynamics calculation shows realistic picture of how the internuclear distance would vary with time in a reaction if we had no initial momentum, while MEP shows what would happen in a specific step if we always had zero momentum (essentially describes how the reaction varies depending on the initial conditions, as shown below.
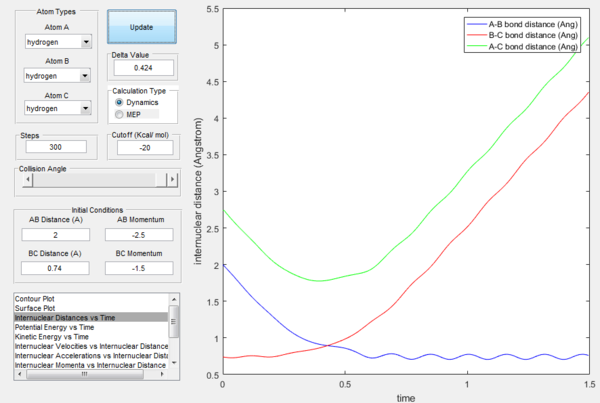
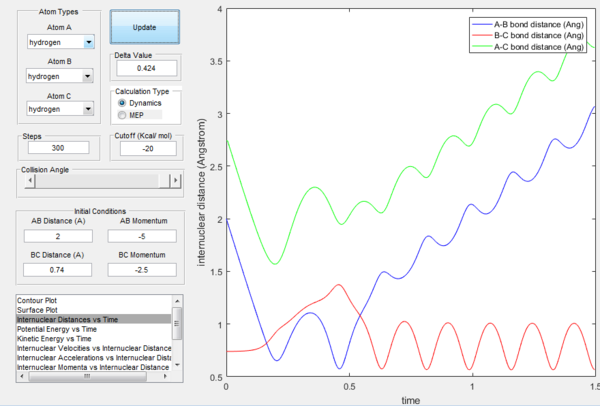
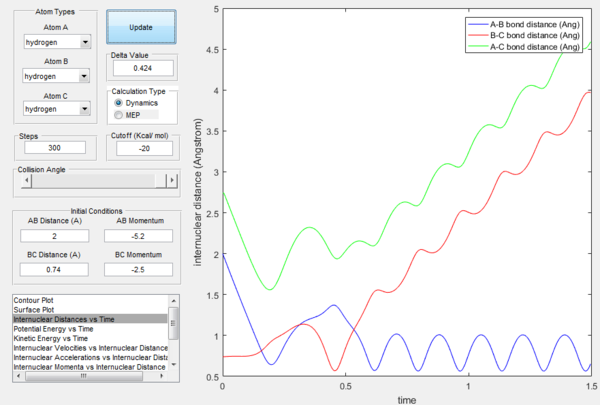
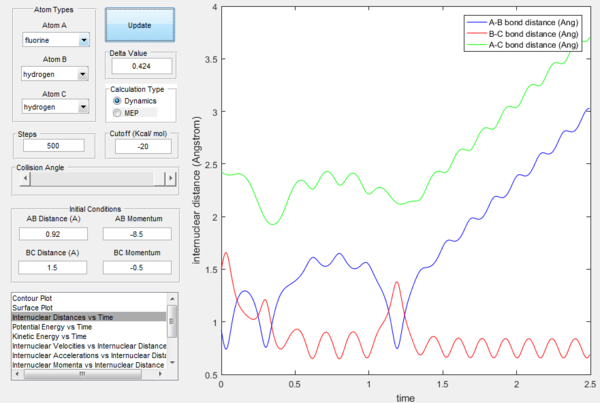
If the momenta are reversed at the end of the experiment, the system passes near transition state and A atom goes free, while B and C form a bond (C was free beforehand).
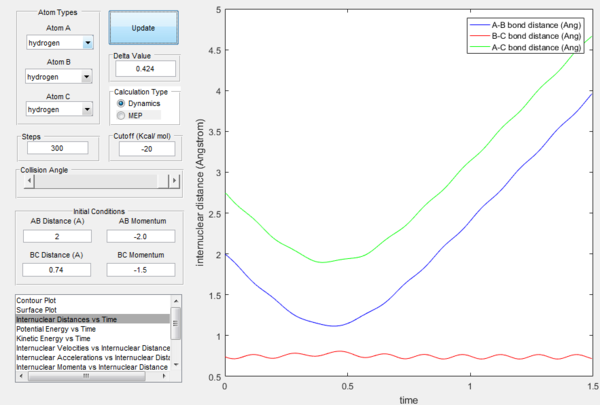
However if we just offset the initial conditions in the opposite direction, the other bond will stay formed (indeed whichever was shorter in the beginning will remain, provided the offset is only small.
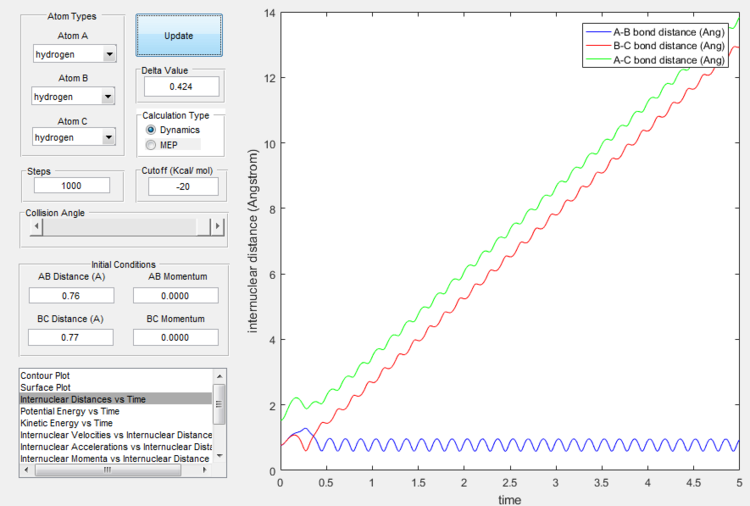
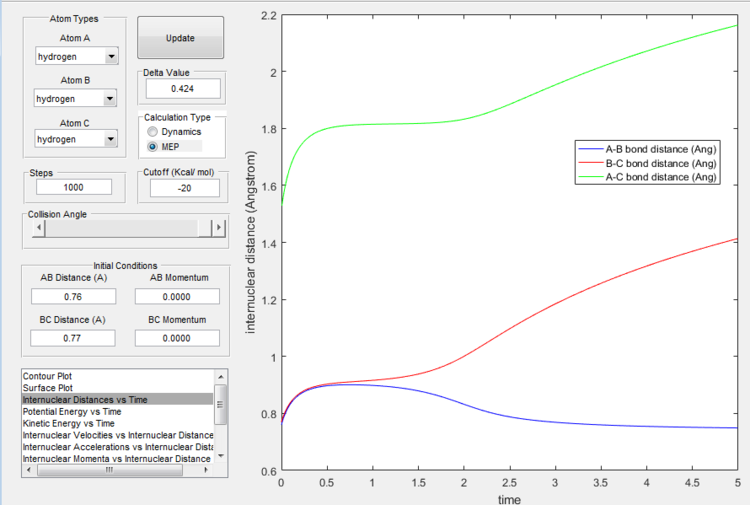
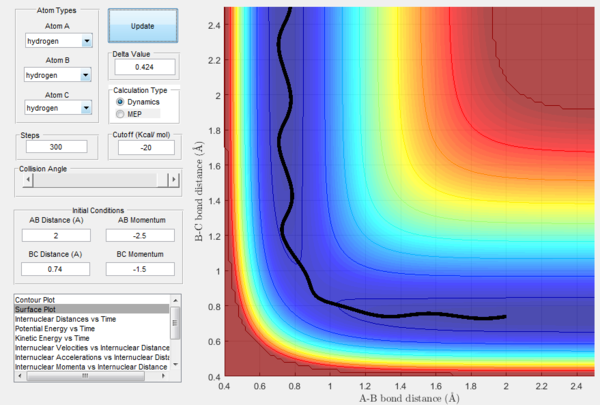
Reactive and unreactive trajectories
The reaction trajectories are shown in table below
Transition State Theory
The main assumption of transition state theory is that there exists a special kind of chemical equilibrium between reactants and transition state. Rate of reactions can be studied by examining activation complexes close to the saddle point (the minimum energy pathway, i.e. transition state). These activation complexes are able to convert to products. [1]
The theory is useful and will quite correctly predict the experimental rates of reaction, but has a few limitations, namely it is poor at describing systems with multi-step reactions, where steps happen in quick succession (because the intermediates do not necessarily have Boltzmann distribution of energies), it does not account for the possibility of quantum tunneling through the activation barrier and does not account for the pathways that do not happen near the transition state saddle point (especially important at very high temperatures). Due to those factors the theoretically obtained value may differ from the experimental values for the reaction rates.
(What does transition state theory say about barrier recrossing? Is it justified based on the results of your simulations? Lt912 (talk) 11:56, 12 June 2017 (BST))
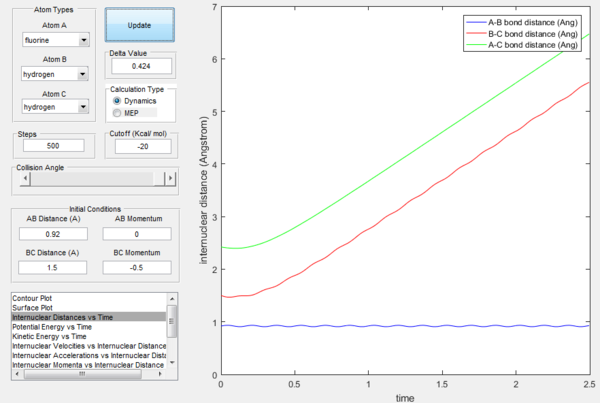
Exercise 2: F - H - H system
H-H bond has smaller dissociation energy (432 kJ/mol) as compared to H-F bond (565 kJ/mol), which means that having a stronger bond between hydrogen and fluorine is favourable energetically, thus F + H2 is exothermic, because it leads into breaking of weaker and forming of a stronger bond in lieu, while HF + H is endothermic (being essentially the first reaction in reverse). H-F bond is evidently significantly stronger.
Finding transition state
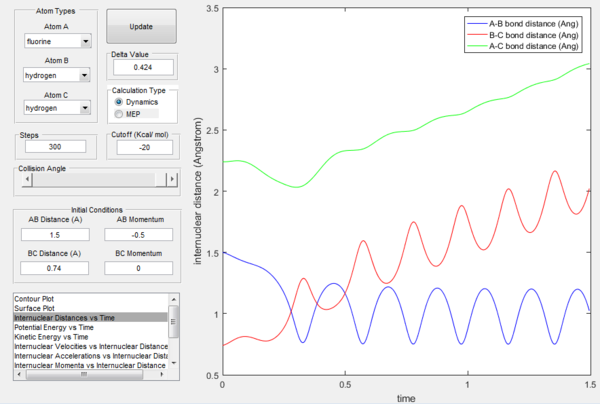
Transition state will occur close to the bond lengths of H-H and H-F, which are 0.74 and 0.94 Å respectively. However, we expect transition state to be closer to F + H2 side, because of their higher energy (Hammond's postulate states the transition state will be closer to whichever state is closer to it energetically). Thus a good starting point would be 0.76 Å for H-H distance (transition state in the previous case), and slowly increasing H-F distance until getting favourable energy picture.
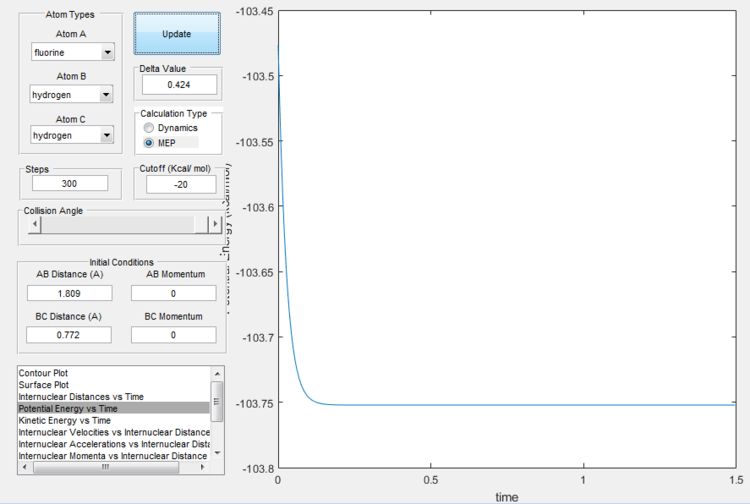
It is found that the transition state is at H-H distance 0.772 Å and H-F distance 1.809 Å, as shown below.
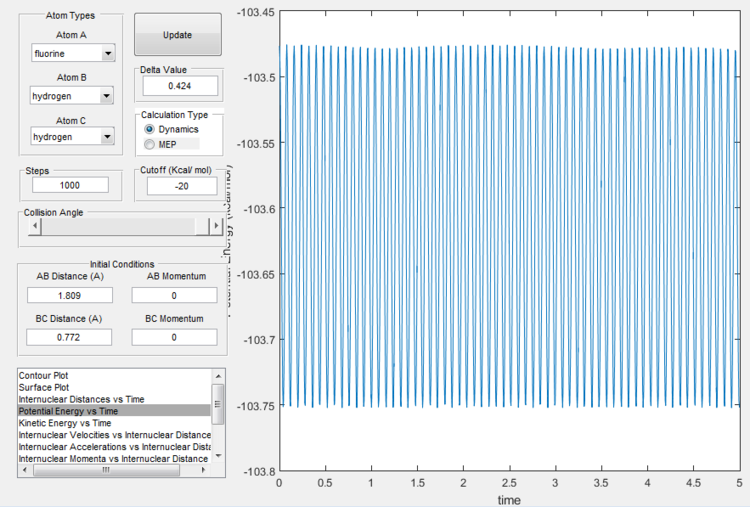
Activation energy
Activation energy for transformation from F + H2 to H + HF side is as little as 1.1 kJ/mol, while the reverse reaction has activation energy of about 126 kJ/mol. Below the energy of transition state, reactants and products is shown.
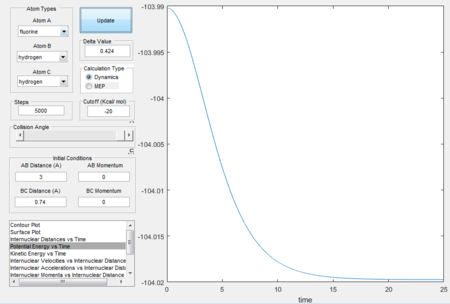

The excess energy
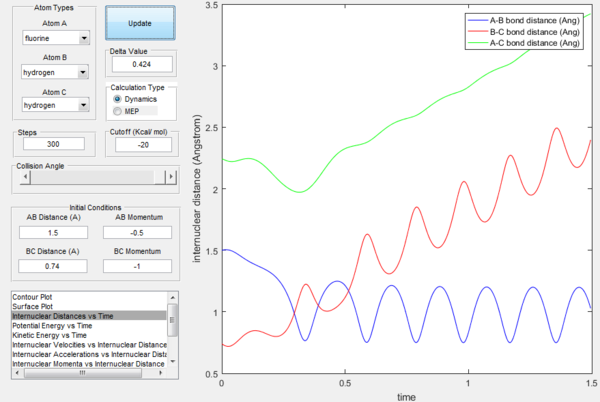
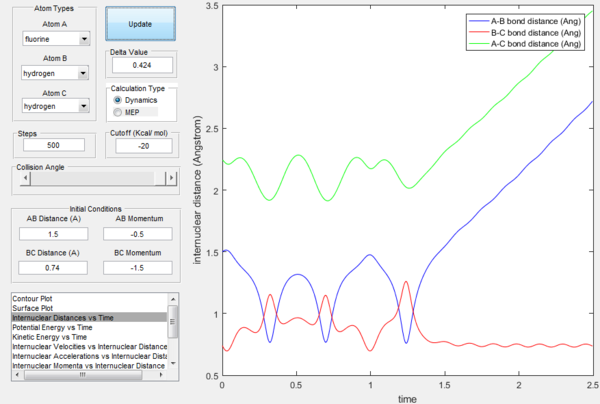
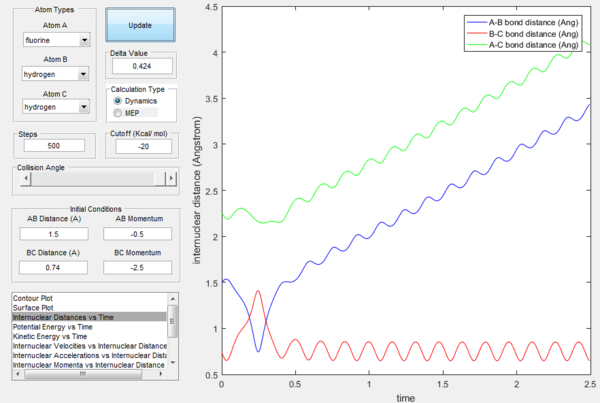
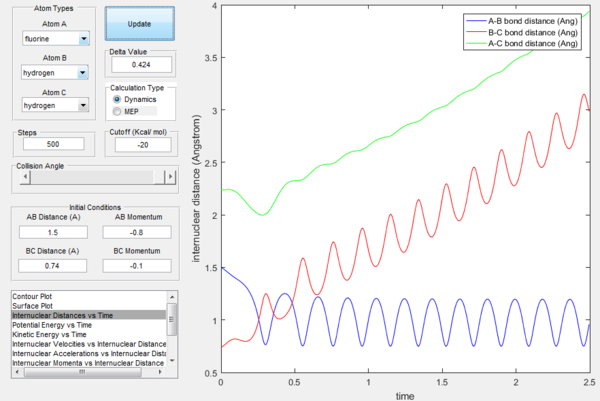
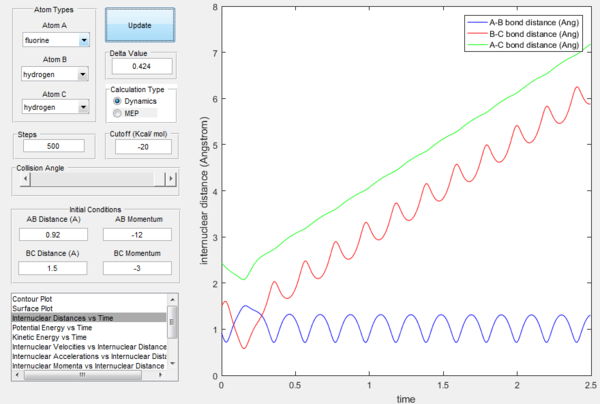
The excess energy that has been produced as a consequence of the reaction of F with H2 is dissipated in the form of vibrational energy as shown in the two figures below (larger variation in intermolecular momentum means larger vibrational energy). The overall translational energy changes less compared to vibrational energy.
(where does this excess energy come from? if energy is conserved and you are using a closed system to where does the energy dissipate? How could this be confirmed experimentally? Lt912 (talk) 12:03, 12 June 2017 (BST))

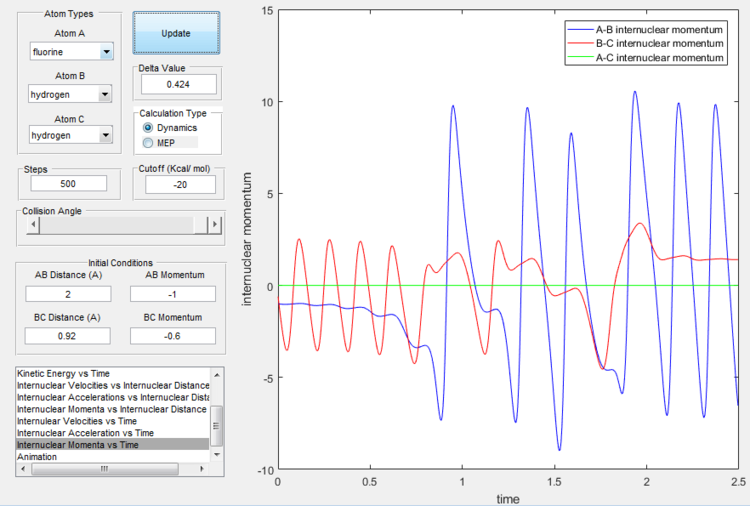
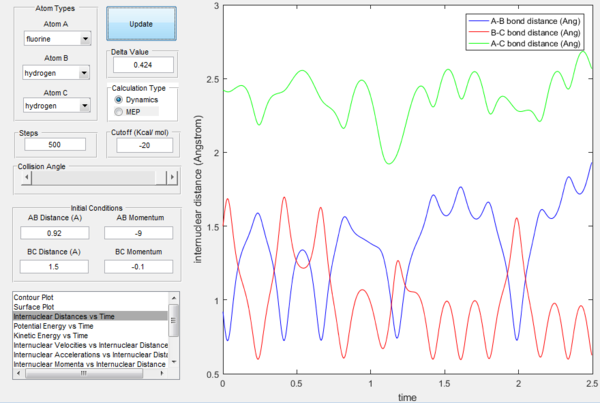
Empirical rule of energy distribution's effect
Polanyi's rules state that when the reactants are closer to transition state, the vibrational energy contribution is significantly less important than translational energy contribution, while the vice-versa is also true, when the products are closer to the transition state, vibrational energy has more significant effect. If the form of energy that is not needed is significantly larger that tends to prevent reaction, as shown in the table below. The sign of momentum of vibration does not create much of a difference.