MRD:ABR01190874
Exercise 1
Defining the Transition State
The transition state is mathematically defined as the saddle point on the potential energy surface. The saddle point is the highest energy point on the reaction coordinate, which is defined as the lowest energy pathway connecting the reactants and products. A saddle point is a point on the surface of a graph of a function where in orthogonal directions, the first derivatives are all 0, and in the plane in the direction of the reaction path, the second derivative is positive, indicating a local minimum, and in the orthogonal plane, the second derivative is negative, indicating a maximum. It can be identified thus and distinguished from a local minimum of the potential energy surface as a local minimum will not fulfil the above conditions related to second derivatives in orthogonal directions. Great answer. Thank you. Mak214 (talk) 17:20, 13 May 2019 (BST)
Locating the Transition State
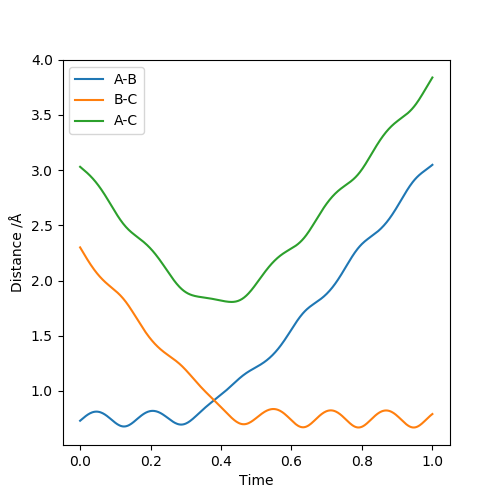
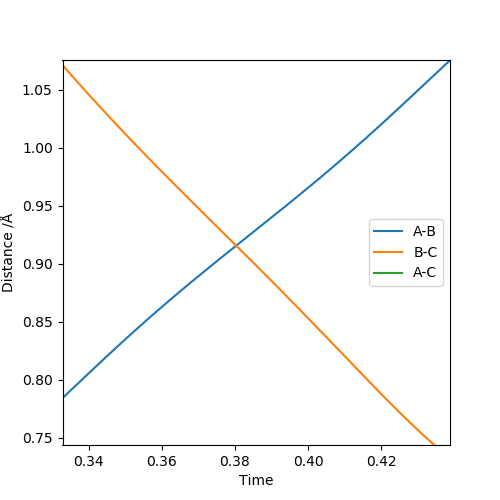
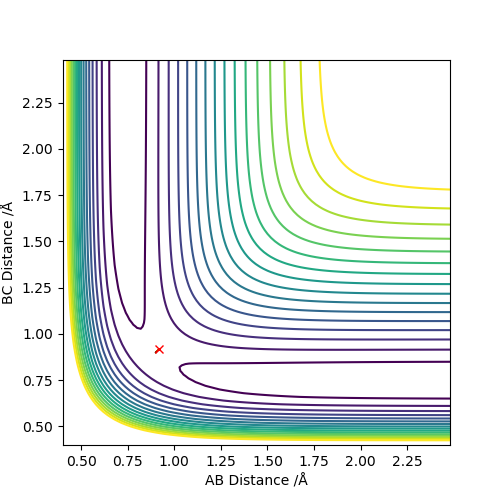
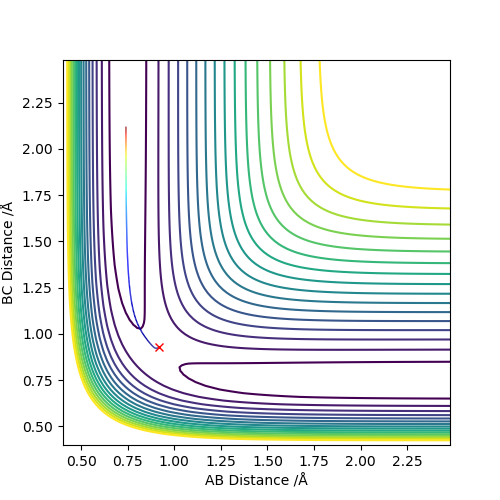
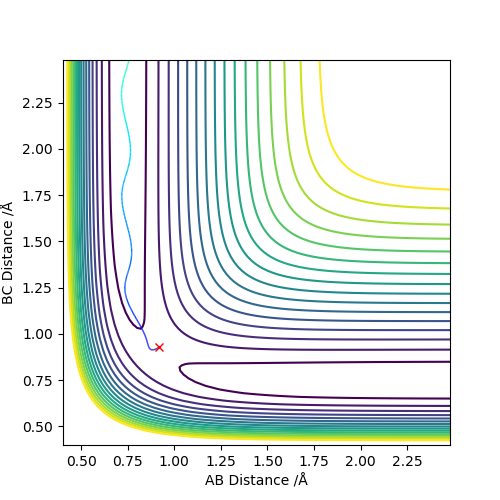
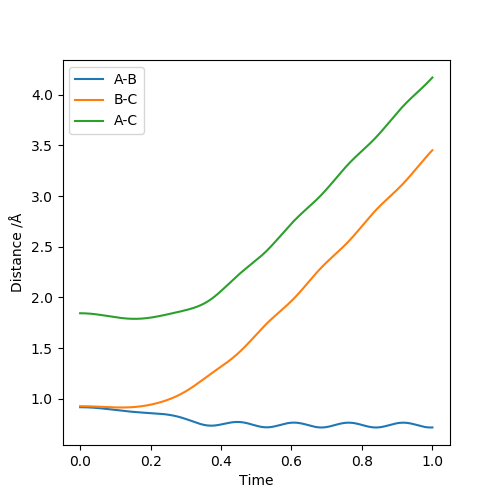
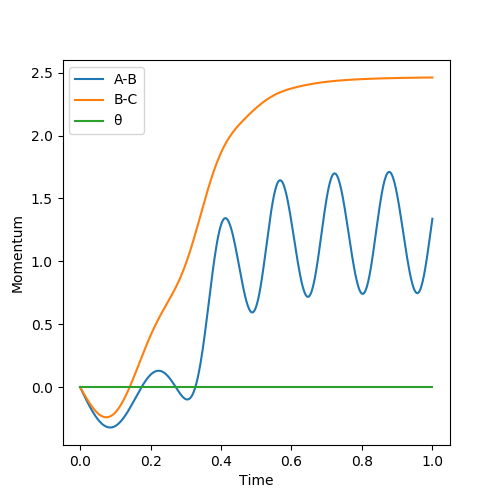
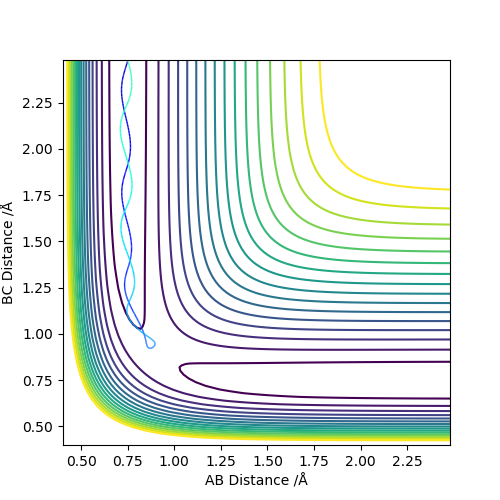
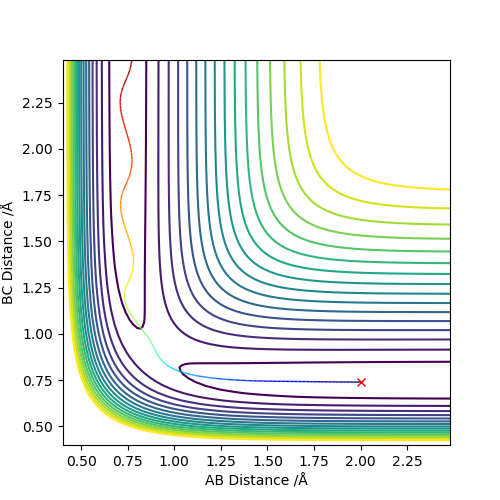
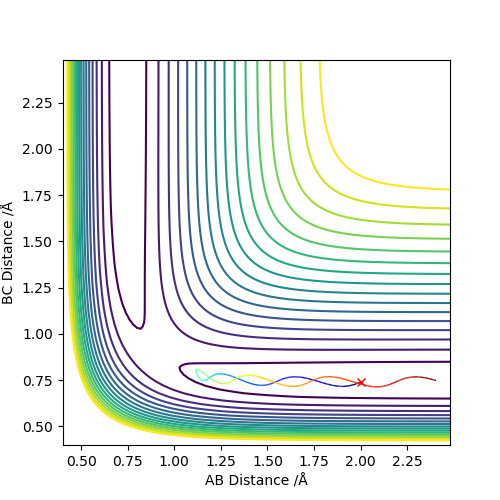
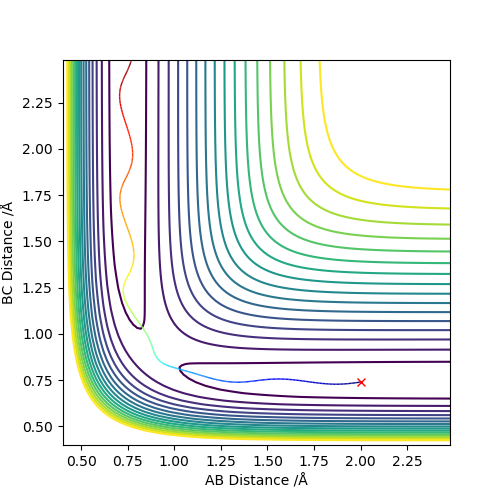
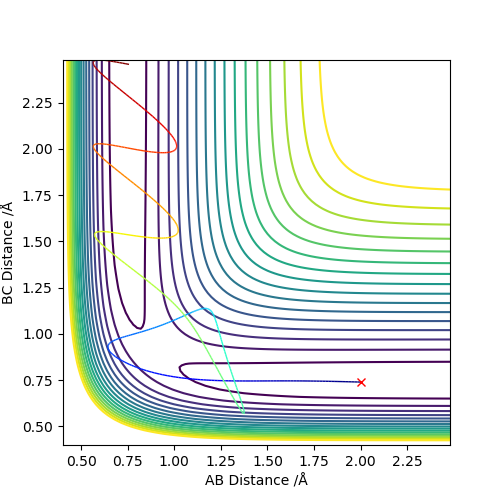
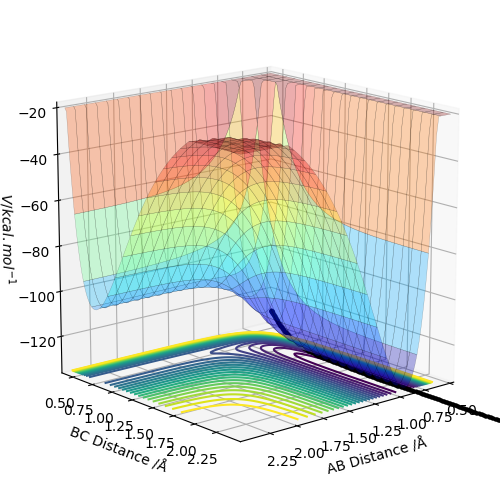
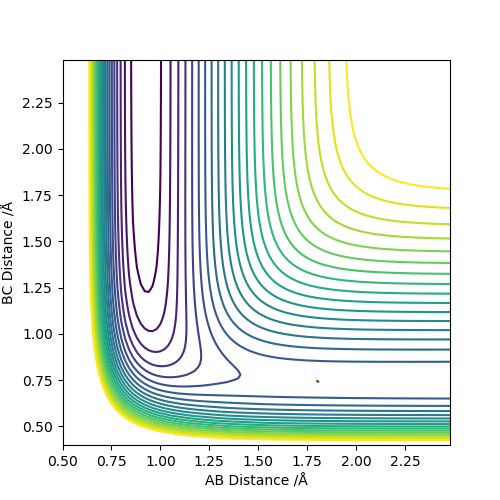
The best estimate of the transition state position (rts) is 0.917. This was found by analysing the "Internuclear Distances vs Time" plot for a reactive trajectory, and finding the point where the lines intersect ie. the distances between all the atoms are the same. Since, in this reaction, the reactants and products are the same, the transition state is symmetrical and occurs when distances AB and BC are the same, and the reactive pathway passes through the transition state. The first figure shown above demonstrates this plot, and the second is a zoomed in version of the intersection. The third plot shown above is a trajectory starting at the transition state with no momenta, and shows that the molecules stay more or less fixed, and no reaction occurs, since at the transition state, if the reactants and products have no momenta they will stay at the transition state (it is an unstable stationary point of sorts). The figure below shows the transition state (marked by the red cross) on a contour plot of the same trajectory. Very good. Mak214 (talk) 17:20, 13 May 2019 (BST)
Calculating the Reaction Path
- Comment on how the mep and the trajectory you just calculated differ.
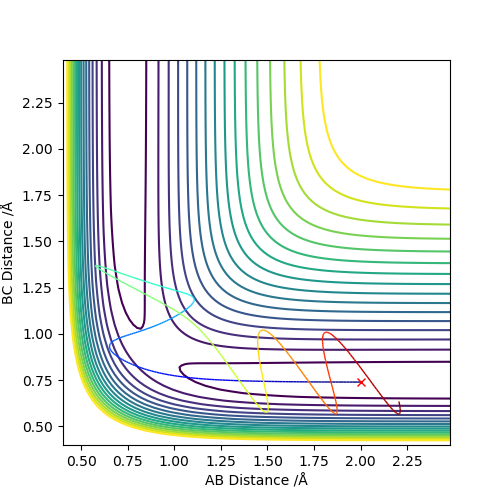
Going from left to right regarding the diagrams below, the top leftmost one shows a contour plot of the MEP. The contour plot directly to the right shows the dynamics calculation with the same initial conditions as the MEP (r1=0.927, r2=0.917, both momenta=0). These differ because the dynamics calculation shows the oscillation of the molecules (ie the internal energy of the particles), whereas the MEP does not since it resets the momenta to zero in each time step. If the calculation were done with r1 and r2 swapped, the reaction would progress in the other direction along the contour plot. The “Internuclear Distances vs Time” and “Momenta vs Time" plots are shown next (from the dynamics trajectory using the same initial conditions as the MEP), and using the final values of position and momenta from these plots for initial conditions, the final plot was created (with the signs of the momenta reversed). This shows the system approaching the transition state, and then reforming the reactants. Good. Mak214 (talk) 17:20, 13 May 2019 (BST)
Reactive and Unreactive Trajectories
From this table, one can conclude that a trajectory having sufficient energy to overcome the activation barrier of a reaction is not enough in and of itself to ensure that a reaction will happen. The correct ratio of vibrational and kinetic energy must also be present. Good. Mak214 (talk) 17:20, 13 May 2019 (BST)
Transition State Theory
Transition state theory makes several assumptions, the most important of which are that the reactants follow the Boltzmann distribution of energies, and that once the once the system reaches the transition state, heading towards the products, it will not go back to the reactants state again. Transition state theory assumes that the energies follow the Boltzmann distribution of energies before reacting. This becomes important when the theory is applied to multi-step reactions, as it assumes that each intermediate is long-lived enough to reach a Boltzmann distribution of energies before continuing to the next step. Some intermediates in some reactions are too short lived for this to happen, and thus transition state theory breaks down in these cases. There are other assumptions made too - it assumes that atomic nuclei behave according to classical mechanics, and that unless atoms or molecules collide with enough energy to form the transition state structure, then no reaction occurs. According to quantum mechanics, there is a possibility that particles can tunnel across barriers (the barrier here being the energy barrier to get to the transition state). Therefore there is a possibility that the molecules will react even if they do not collide with enough energy to cross the barrier. Tunnelling probability decreases with barrier height, and thus for reactions with a low activation energy this becomes important. Lastly, transition state theory assumes that the reaction system will pass over the lowest energy saddle point on the potential energy surface (defined as the transition state), which is not necessarily the case for some reactions at elevated temperatures. [1]
Due to these assumptions, it is likely that the predicted reaction rate will be greater than the experimental values, due to the fact that Transition State Theory assumes that the transitions state will not reform the reactants; in reality this can occur and will cause the rate to be slower than predicted. Although tunnelling would theoretically increase the rate compared to the predicted rate, this is likely to be a very small deviation and would be outweighed by the experimental rate being slower due to the transition state reforming the reactants.Very good. Well done. Mak214 (talk) 17:20, 13 May 2019 (BST)
Exercise 2
Potential Energy Surface Inspection
The surface plot above shows a reaction which results in the formation of a molecule of HF from F + H2. From this, it can be seen that the products of this reaction are much lower energy than the reactants, and thus the F + H2 reaction is exothermic. The H + HF reaction is simply going along the potential energy surface in the opposite direction (ie. in this case the reactants would be lower energy than the products) and thus this reaction is endothermic.The HF bond is a strong one (565 kJmol-1) compared to H2 (436 kJmol-1), and thus it makes sense that a reaction which breaks a weaker bond and forms a stronger one (F + H2) is exothermic, since bond breaking is endothermic and bond making is exothermic, and vice-versa. Good. Please remember to reference where you got your Bond enthalpies from in future! Mak214 (talk) 17:24, 13 May 2019 (BST)
Since the activation energy of the F + H2 reaction is so small, identifying the position of the transition state from the potential energy surface is difficult; however, the Hammond postulate states that the structure of the transition state resembles whichever of the reactants or products it is closer to in energy. In this case, the transition state very closely resembles the reactants, and this information can be used to determine its exact structure, by starting with a structure very close to the reactants and using trial and error. In the below contour plot showing the transition state, the HF distance is 1.809 and the HH distance is 0.74.
In order to determine the activation energies, an MEP calculation was performed using a trajectory slightly displaced from the transition state (HF distance 1.6 and HH distance 0.74). An energy vs time plot (shown below) was used to calculate the activation energy for the endothermic reaction (HF + H), as it is the height of the 'step' seen. This was calculated to be 29.18. The activation energy of the exothermic reaction (F + H2) is so small it can be considered to be 0, as shown by the fact that the transition state structure is essentially the reactants. Good. Mak214 (talk) 17:24, 13 May 2019 (BST)
Reaction Dynamics
Energy is conserved, and the F + H2 reaction is exothermic. The excess energy released during the reaction is converted to vibrational energy of the HF molecule and kinetic energy of the HF molecule and the H atom. This could be confirmed experimentally - calorimetry could be used to measure the kinetic energy (heat) released, and emission spectroscopy could be used to measure the vibrational energy of the HF molecule. The energies could be added to show conservation of energy, as the difference in energy between reactants and products would be the sum of the extra vibrational and kinetic energy of the products. Great. Mak214 (talk) 17:24, 13 May 2019 (BST)
For an exothermic reaction, the transition state is near reactants (an early transition state,close in energy ); for an endothermic reaction, the transition state is near products (a late transition state). According to the Hammond postulate, species with similar energies have similar structures, and thus an early transition state resembles the reactants, and a late transition state resembles the products. The Polanyi rules state that vibrational energy is more efficient to promote the success of a late transition state reaction, and translational energy is more efficient for a reaction with an early transition state. Therefore, for the H2 + F reaction, a larger proportion of translational energy is needed than for the HF + H reaction, which needs a larger proportion of vibrational energy. [2] Spot on report thank you. Mak214 (talk) 17:24, 13 May 2019 (BST)
References
- ↑ Atkins, P., De Paula, J. and Keeler, J. (2009). Atkins' physical chemistry. 5th ed. Oxford University Press.
- ↑ Zhang, Z., Zhou, Y., Zhang, D., Czakó, G. and Bowman, J. (2012). Theoretical Study of the Validity of the Polanyi Rules for the Late-Barrier Cl + CHD3 Reaction. The Journal of Physical Chemistry Letters, 3(23), pp.3416-3419.