MRD:3008
EXERCISE 1: H + H2 system
On a potential energy surface diagram, how is the transition state mathematically defined? How can the transition state be identified, and how can it be distinguished from a local minimum of the potential energy surface?
A transition state represents a maximum, in the minimum energy reaction path linking the reactants and products.
Mathematically it is defined as a saddle point and essentially depends on two parameters rab and rbc.
This mathematically means that the following conditions need to be satisfied:
1)Vx=0 and Vy=0
2)VxxVyy-(Vxy)2<0
where x=rab and y=rbc.
Although a local minimum or a local maximum would satisfy condition 1 , they would not satisfy condition 2, and this is what
differentiates them from the transition state.
Ng611 (talk) 16:48, 7 June 2019 (BST) Good.
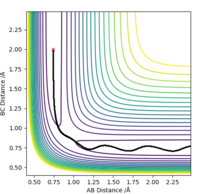
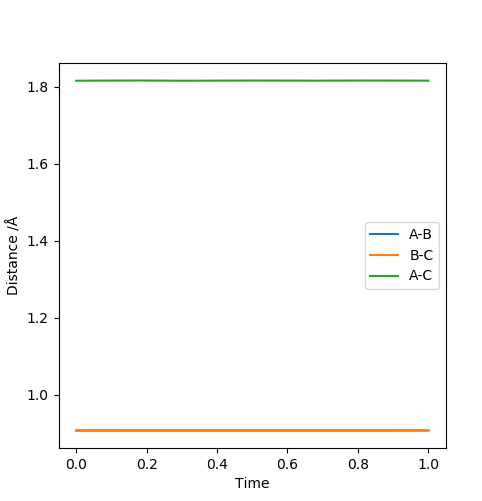
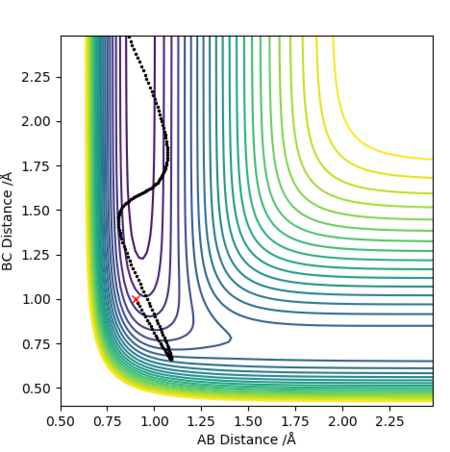
Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” plot for a relevant trajectory.
The transition state geometry was found in a trial and error process and the optimal distance was
recorded to 4 d.p. Unsurprisingly the transition state was found to be symmetrical with an equal
distance between the atoms, rts=0.9076 Å.
The “Internuclear Distances vs Time” plot illustrates this very well, since as it can be seen that
a straight line graph is obtained for both rab and rbc and not an oscillating
one. This indicates that the distances remain unchanged over time and that there is no kinetic energy
i.e. all is potential.
Ng611 (talk) 16:48, 7 June 2019 (BST) Good.
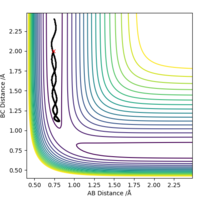
Comment on how the mep and the trajectory you just calculated differ.
The MEP trajectory is smoother than the MD trajectory and thus is due to the way MEP works.
Any deviation from the transition state will result into the atoms 'rolling down' the potential
surface and of course gain momentum as that happens. In MEP before each step the momenta are set
to zero, so the momentum gained by rolling down the PES is not taken into account. In both cases
the distance between the initially slightly displaced H atom increases as it moves away from the
H2. But , the MD calculation shows that indeed the atoms just just simply more, they also oscillate
in the molecule following the curvature of the PES in that region. On the other hand, as explained
above the MEP calculation yields a smooth non-oscillating path.Also note that the free H atom in MEP move
a shorter distance since any momentum gained in MD rolling down the PES is absent here.


Complete the table above by adding the total energy, whether the trajectory is reactive or unreactive, and provide a plot of the trajectory and a small description for what happens along the trajectory. What can you conclude from the table?
It can be concluded from the table that not any combination of momenta will result into products. The relative distribution of the momenta between atom and molecule will affect the reactivity. Hence whether a lot of energy is given to the atom colliding with the diatomic or whether the diatomic itself possesses a lot of vibrational energy affects the reactivity. Not any combination of momenta in the region stated in the script will work. Also as it can be seen from reaction 4, excessive kinetic energy will not necessarily lead to successful reaction.
State what are the main assumptions of Transition State Theory. Given the results you have obtained, how will Transition State Theory predictions for reaction rate values compare with experimental values?
Transition state theory relies on three key assumptions in its derivation.(Levine Physical Chemistry 6th Edition)
- Reactants are in constant equilibrium with the transition state structure.This reaction exists as an equilibrium because we assume that not every collision results in the formation of the transition state. The energy of the particles follow a Boltzmann distribution.
- Once reactants cross the transition state hyperspace, the transition state structure does not collapse back to the reactants.
Ng611 (talk) 16:49, 7 June 2019 (BST) What are the other assumptions? There are several you missed out.
The last assumption in the one that is more relevant to the calculations above. As seen in reaction 4 , this is clearly going against the assumption, as it illustrates a case of barrier recrossing. In this case even though the reactants became the transition state, they collapse back to the reactants, making the initial conditions unsuitable for a successful reaction.As a result, ignoring the effect of recrossing would over estimate the reaction rate for some reactions, such as this one.
It is important to note that the transition state theory assumes that the reactants actually have to follow the reaction trajectory and pass through the transition state point. However in reaction 4,quantum mechanical tunnelling goes against it. Additionally, multiple TS recrossing just like in reaction 5 might still lead to an overall successful reaction, but the time taken is will be greater. Thus if this effect is also ignored, just like above, the reaction rate will be over estimated.
Ng611 (talk) 16:51, 7 June 2019 (BST) TS recrossing that still results in a reaction has no net effect. More important are the ones that result in product reformation.
EXERCISE 2: H-F-H system
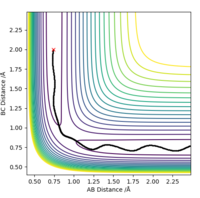
By inspecting the potential energy surfaces, classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved.Locate the approximate position of the transition state.
As it can be seen from the potential energy surface plot below, the reaction F + H2 ----> H + HF is exothermic and releases energy, since the products are lower in potential energy than the reactants. This of course means that the backwards reaction is endothermic.
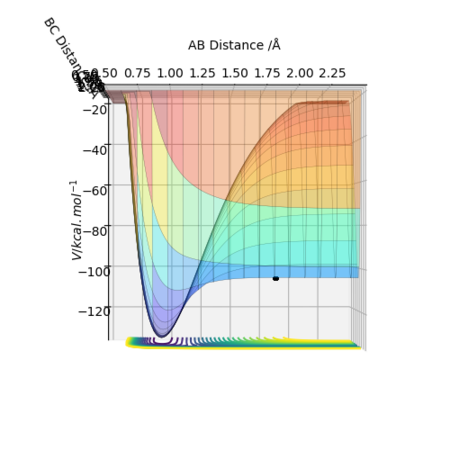
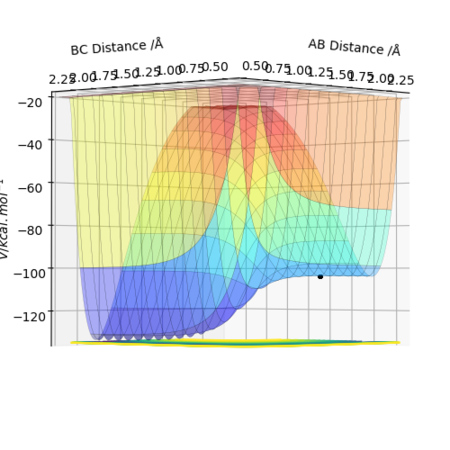
This implies that the H-F bond is stronger than the H-H bond, thus when the reaction process in the defined forwards
direction energy is released to the environment.
The approximate coordinates of the Transition State F-H-H are, rF-H=1.8127 Å and rH-H=0.7443 Å.
Initially an approximate guess was made by simply examining the energy contour plot to locate a starting point. Then values
close to the initial guess we inputted in the calculation until a transition state was obtained. This was confirmed by an
animation where all three atoms were not moving.
Report the activation energy for both reactions.
To obtain the thermodynamic data for both reactions the reactant and product system had to be studied. By choosing appropriate parameters of the system with geometries on 'either side' of the transition state barrier and using the transition state energy(-103.752 kcal/mol) the energies of reactants and products were obtained.


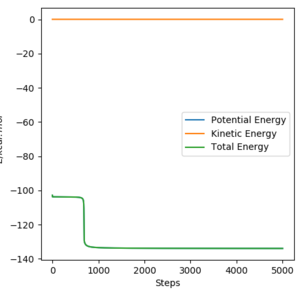
This allowed for the calculation of ΔE of the reaction and the activation energies:
1)F + H2 ----> H + HF
- ΔΕ=-29.558 kcal/mol
- Ea= +0.249 kcal/mol
2)H + HF ----> F + H2
- ΔΕ=+29.558 kcal/mol
- Ea= +29.807 kcal/mol
Ng611 (talk) 16:51, 7 June 2019 (BST) Good.
In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. Explain how this could be confirmed experimentally.
As stated above the reaction F + H2 ----> H + HF is an exothermic reaction. However the excess energy of the reaction is not wasted but conserved. This energy is converted in vibrational energy in the H-F bond and it can be seen quite distinctly by the large amount of oscillations in the contour energy plot, indicating high degree of vibration. Furthermore the plot of Energy vs Time and Momenta vs Time clearly shows that the large interchange of kinetic and potential energy and the large increase in the H-F bond momentum after the reaction. Both are indicative of the increase in vibrational energy in the bond.


In the large scale, one potential way to measure this increase in vibrational energy could in principle FTIR spectroscopy by monitoring the decay of the vibration through the course of time.
Ng611 (talk) 16:53, 7 June 2019 (BST) What features of the IR spectrum would allow you to determine vibrational excitation?
Alternatively another way could be calorimetry since eventually this excess vibrational energy is dissipated in the surroundings, raising the temperature of water in a calorimetric measurement(an exothermic reaction!)
Ng611 (talk) 16:53, 7 June 2019 (BST) Not enough on its own as it doesn't distinguish between vibrational and translational excitation.
Discuss how the distribution of energy between different modes (translation and vibration) affect the efficiency of the reaction, and how this is influenced by the position of the transition state.
The Polanyi rules, which state that vibrational energy is more efficient in promoting a late-barrier reaction
than translational energy.(Z. Zhang ,Y. Zhou, D. H. Zhang, G. Czakó, J. M. Bowman,J. Phys. Chem. Lett.2012,3,23,pp3416-3419)
A late barrier reaction, by the Hammond postulate effectively means an endothermic reaction, and in this case the H + HF ---->H2+ F system.
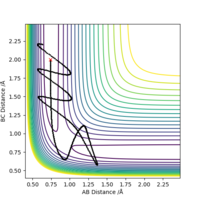
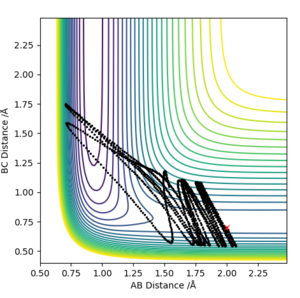
A reaction with initial parameters pHF=0.1, rHF=0.9 Å, pHH=-5, rHH=1 Å , did not prove to be a reactive trajectory, as it can be seen from the contour plot below
More over, increasing the momentum, in excessively high amounts above the activation energy did not yield a successsful reaction. Showing that the activation energy is not the only criterion that need to be met.
To test the rule above, the reaction was repeated with initial parameters pHF=5, rHF=0.9 Å, pHH=0, rHH=1 Å. As expected that indeed gave a successful reaction as shown by the second contour plot.
So as Polanyi rightly states, for the endothermic reaction, it is much more efficient to have the required activation energy in the form of vibrations, ie high values of pHF, rather than high values of translational energy of the incoming atom ie high pHH.

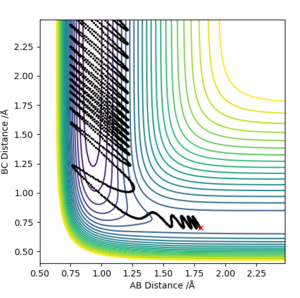
To examine the validity of these rule in an early transition state, the reverse exothermic reaction F+ H2 ----> H + HF was tested.Initially the set system was set up as pHF=-0.5, rHF=2 Å, pHH=-5, rHH=0.74 Å. This did not yield a successful reaction, despite having enough energy in the form of vibration in the H-H bond. Decreasing the momentum of bond vibration first to pHH=-2 and then to pHH=2, gave a successful reaction in both cases, illustrating that indeed a lower energy in vibration is more effective in the conversion of reactants to products in this endothermic reaction.
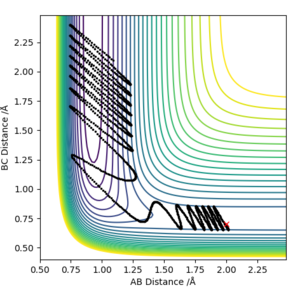

Furthermore, varying the amount of momentum of the colliding F atom from pHF=-1 to pHF=-2 and pHF=-3, with initial parameters rHF=2 Å, pHH=0, rHH=0.74 Å, further complies with the rules. For pHF=-1 it was unreactive but as the momentum of F atom increased to -2 and -3, the pathways became reactive, showing that indeed for exothermic reactions the best way to get a successful conversion is to put the energy in translational motion and not vibrations.



Ng611 (talk) 16:55, 7 June 2019 (BST) Good H+HF trajectories, but your F+H2 trajectories don't demonstrate polanyi's rules as you've started part way up the activation barrier!.