MRD:24970
H2 Molecule
Dynamics from the transition state region
On a potential energy surface diagram, how is the transition state mathematically defined? How can the transition state be identified, and how can it be distinguished from a local minimum of the potential energy surface?
The transition state is defined as the maximum point on the lowest energy path between reactants and products where the first partial derivative (∂V/∂r) is equal to 0 and the second derivative, (∂V2/dr2) , is positive. It can be distinguished from a local minimum as it satisfies the condition that ∂V/dq1 = 0 and ∂V/dq2 = 0, where q1 is the tangent at the minimum of a reaction pathway and q2 is a line at right-angles to this tangent.
This isn't quite right mathematically. As the transition state is the maximum point on the lowest energy pathway, the second partial derivative in the direction of the lowest energy pathway is negative, as the second partial derivative of a maximum is negative. As the transition state is a point on the lowest energy pathway, it is a minimum in the direction that is orthogonal to the reaction trajectory and therefore in that direction the second partial derivative is positive because the second partial derivative of a minimum is positive. Rk2918 (talk) 10:45, 28 May 2019 (BST)
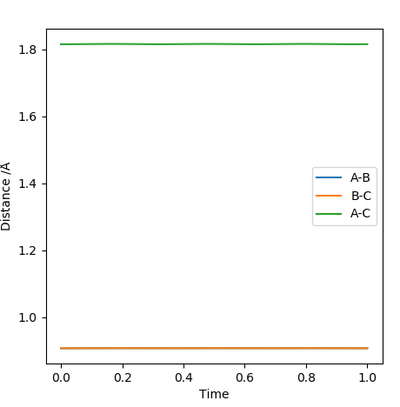
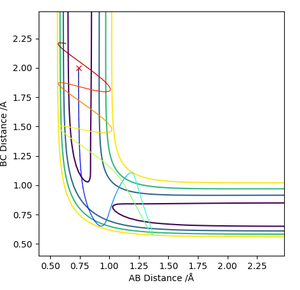
Report your best estimate of the transition state position (rts) and explain your reasoning, illustrating it with a “Internuclear Distances vs Time” plot for a relevant trajectory.
The best estimate for the transition state position is where r1 and r2 are 0.9075Å because at this inter-atomic distance, the atoms are static and not vibrating. In the Internuclear Distance vs Time plot, the distance remains the same over time.
I can see what you are getting at but you need to provide a much more in depth explanation of what you are trying to say. For example, you need to explain why atoms not vibrating means that you have found the TS. The reason is that as the molecule approaches the TS (increases in E on the potential surface) it gains potential energy and therefore it must lose vibrational energy due to conservation of energy. Again, what do you mean by the internuclear distances remaining the same over time? Specify a time frame, and specify why this means you are at the TS. Rk2918 (talk) 10:45, 28 May 2019 (BST)
Comment on how the mep and the trajectory you just calculated differ.
The trajectory calculated by MEP differs from the one calculated by Dynamics as it does not oscillate, whereas the one calculated by Dynamics does. This means that the H2 molecule is vibrating as it moves away from the hydrogen atom , whereas in the MEP it is not. Another difference is that in the MEP calculation , the trajectory stops at the r1 distance of 2.25Å whereas it continues to infinity in the Dynamics calculation.
But why does the MEP not oscillate but the Dynamics calculation does? Do you understand the difference between them on the level of the calculation? This is important to demonstrate. Rk2918 (talk) 10:45, 28 May 2019 (BST)
Reactive and Unreactive Trajectories
One can conclude from the above table that the total energy of a reaction is determined by the momentum of the reacting particles, which is related to their kinetic energies. However, the success of the reaction is determined not by the total energy of the reaction, but by the relative momentum values of p1 and p2.
This is a fairly insightful conclusion. Rk2918 (talk) 10:51, 28 May 2019 (BST)
State what are the main assumptions of Transition State Theory. Given the results you have obtained, how will Transition State Theory predictions for reaction rate values compare with experimental values?
Transition theory states that in a reaction between A and B molecules, an activated complex C is formed that is in equilibrium with the reactants and is at a greater energy than both reactants and products. The prediction of reaction rate by Transition State Theory will be a greater value than that predicted by experiment as it is shown in the penultimate reaction in the table that a reaction can be unsuccessful if barrier recrossing occurs, even if the reacting particles have an energy greater than the activation energy.[1]
The discussed theory is called transition state theory, not transition theory. You have partially answered the question but you were asked to state 3 assumptions made by the theory. Rk2918 (talk) 10:51, 28 May 2019 (BST)
Exercise 2: F-H-H System
By inspecting the potential energy surfaces, classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved?
The F + H2 reaction is exothermic as the potential surface shows that the products have a lower energy than the reactants. Hence, the reverse reaction to this ( H + HF) must be endothermic as the products are higher in energy than the reactants. The process of bond making is exothermic and as the reaction of F and H2 to form HF is exothermic and this involves making a H-F bond and breaking a H-H bond, we can conclude that the H-F bond is stronger than the H-H bond.
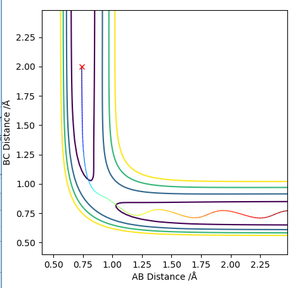
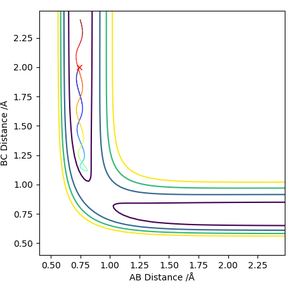
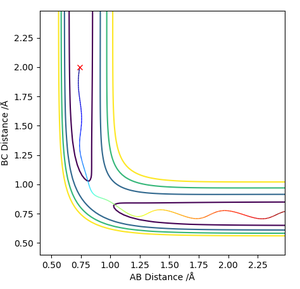
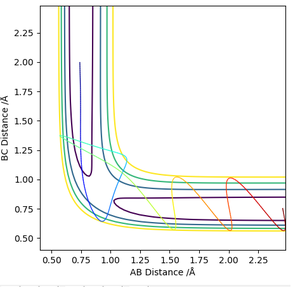
Locate the approximate position of the transition state.
The H-H distance in the transition state is 0.745 Å while the H-F distance is 1.808 Å. The contour plot at these inter-nuclear distances show that there is no reaction trajectory.
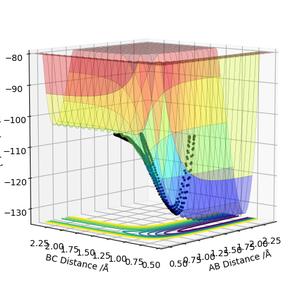
Report the activation energy for both reactions.
The activation energy for the reaction H2 + F → HF + H is 0.2 kcal/mol as the transition state has an energy of -103.742 kcal/mol and the reactants have an energy of -103.911 kcal/mol. For the reaction HF + H → H2 + F, the activation energy has a value of 30 kcal/mol as the reactants lie at -133.864 kcal/mol.
Where did you get these numbers? It is important in questions like this that you demonstrate your understanding by showing how you find energies etc. Rk2918 (talk) 10:56, 28 May 2019 (BST)
In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. Explain how this could be confirmed experimentally.
The mechanism involves translational energy being converted to vibrational energy, upon collison between the molecule and atom. To measure the release of vibrational energy, an infa-red spectrum could be experimentally recorded whereas the translational energy could be measured using bomb calorimetry, which provides a measure of the kinetic energy[1].
What exactly does calorimetry measure and how can that be applied in terms of energy? Rk2918 (talk) 10:56, 28 May 2019 (BST)
| pHH value | Reactive? | H-H Bond Vibration Level |
|---|---|---|
| -3 | No | Very Strong |
| -2.95 | No | Strong |
| -2.9 | No | Strong |
| -2.85 | No | Strong |
| -2.5 | No | Strong |
| -2 | Yes | Medium |
| -1.5 | Yes | Medium |
| -1 | Yes | Weak |
| -0.5 | Yes | Very weak |
| 0 | Yes | Very weak |
| 0.5 | No | Very weak |
| 1 | No | Weak |
| 1.5 | No | Medium |
| 2 | No | Medium |
| 2.5 | No | Strong |
| 2.85 | Yes | Strong |
| 2.9 | Yes | Strong |
| 2.95 | Yes | Strong |
| 3 | No | Strong |
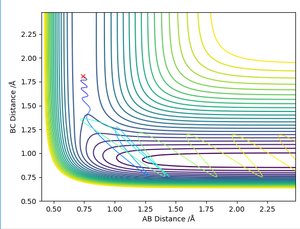
This table shows how changing the momentum of the hydrogen molecule pHH has effect on whether the reaction H2 + F → HF + H will occur. The values of pHF is -0.5 and the H-H and H-F distances are 0.74 and 1.81 respectively. The reaction is successful when the momentum value is between 0-2, and 2.85 and 2.95. This shows that there isn't an obvious relationship between the momentum value and whether the reaction will be successful.
When pHH is changed to 0.1 and pHF is changed to -0.8, it is observed that the reaction is still successful.
Discuss how the distribution of energy between different modes (translation and vibration) affect the efficiency of the reaction, and how this is influenced by the position of the transition state.
Polanyi’s empirical rules state that for an early transition state, like in an exothermic reaction, translational energy has a greater effect than vibrational energy on the efficiency of reaction. On the contrary, endothermic reactions that have a late transition state depend more on the vibrational energy.[2]
Try to be more explicit: in an early TS system, high translational energy will make the system reactive and high vibrational will not, and vice versa. Rk2918 (talk) 10:56, 28 May 2019 (BST)
Due to the exothermic nature of the reaction F + H2 → HF + H, this suggests that the efficiency of the reaction depends more on the translational energy. The H-F momentum represents the translational energy while the H-H momentum represents the vibrational energy. Hence it its predicted that increasing the H-F momentum will have a greater effect in making the reaction go to completion whereas increasing the H-H momentum would have the opposite effect. The table above demonstrates that as the pHH was increased from -1 and -3, the reaction became unsuccessful as there was an increase in H-H vibrational energy.
References
- ↑ Peter Atkins. Judio De Paula. Atkins Physical Chemistry (Ninth Edition). Oxford: Oxford University Press; 2001.
- ↑ 2) J.C.Polanyi. Energy Distribution Among Reagents and Products of Atomic Reactions. J. Chem. Phys. 31, 1338 (1959). Available from: https://doi.org/10.1063/1.1730597 [Accessed 20th May 2019]