MRD:19970410
H-H + H System
Transition state location
1.What value do the different components of the gradient of the potential energy surface have at a minimum and at a transition structure? Briefly explain how minimum and transition structures can be distinguished using the curvature of the potential energy surface.
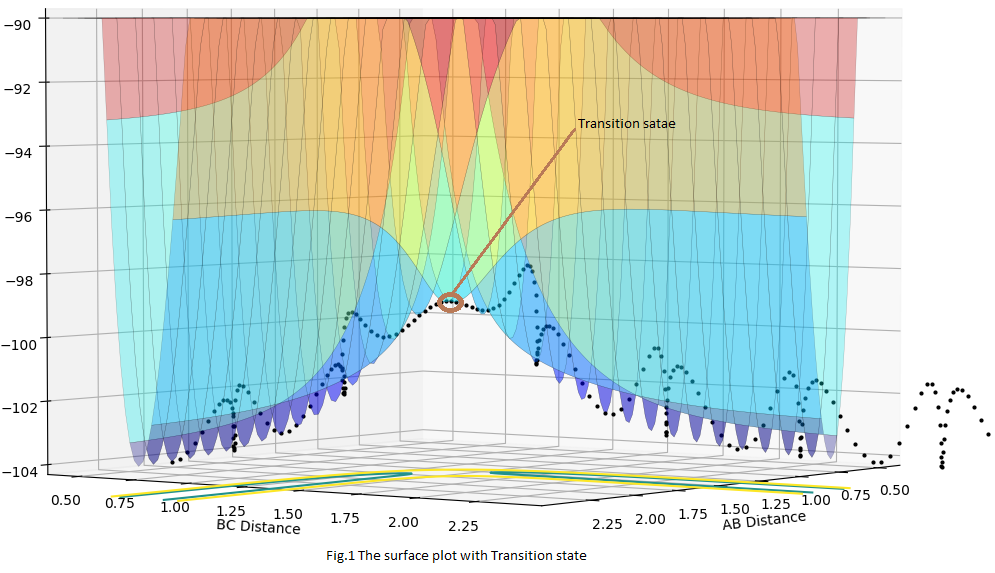
The transition state is the saddle point of the potential energy surface. Firstly, it is the local maximum point of the total energy curve then it is also the local minimum point of the potential energy curve. From Fig.1, the saddle point needs to satisfy both the requirements and was shown the Fig.1. By looking the trajectories (black dotted line), the transition point should be the local maximum point; by looking the potential energy curve ( parabola curve ), the saddle point should be the local minimum point.
At the same time, for this 3 Hydrogen atoms collision, the transition state should be formed when rAB has the same distance with rBC, thus the point should also fulfil this requirement. This can be used as additional evidence.
Before distinguishing the minima and transition state, let us define Q1 and Q2 coordinates in the skew plot.
with
where β is the so-called Skew angle setting the angle of the oblique mesh.
After defining the coordinates Q1 and Q2, derivatives can be used to distinguish the minima and transition state. For both the minima and transition state, their first derivatives need to equal 0:
dV/dQ1 = dv/dQ2 =0
But for the minima,
d2V/dQ2 >0 for all Q
For a transition state,
d2V/dQ2 >0 for all Q, and
d2V/dQ2 <0 along the reaction coordinate.
Very detailed answer. If you are this confident with your answers, why did you not use a proper report style (with intro and conclusion) instead of a lazier QA style. I really like your plots.
2.Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with an “Internuclear Distances vs Time” plot for a relevant trajectory.
The best estimate transition state position is r =0.908 Å.
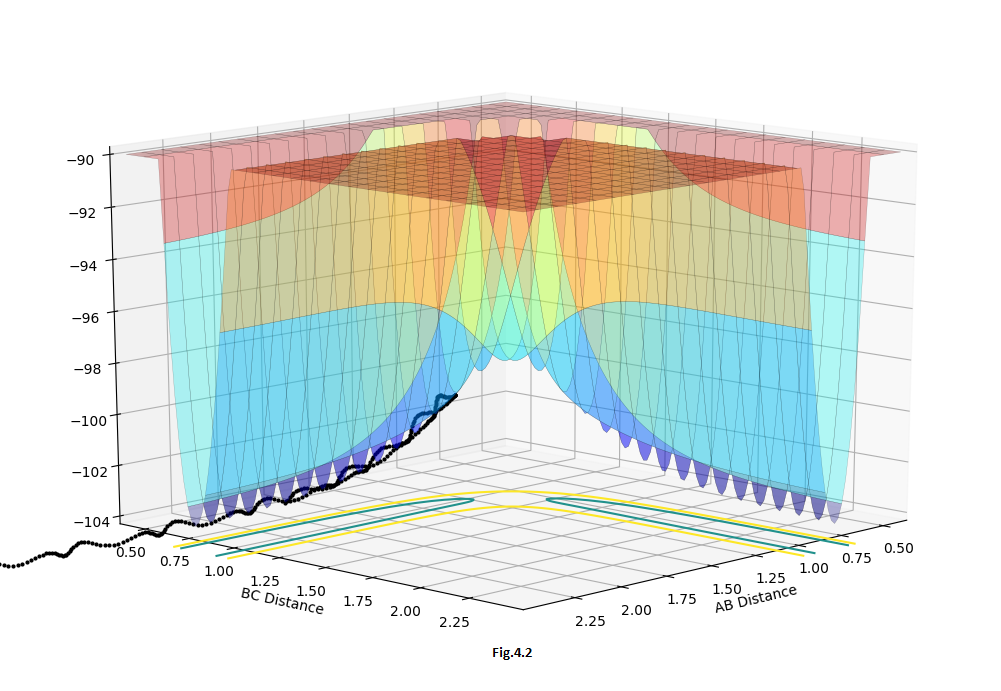
For this symmetrical H+H2 system, the distance between r1 and r2 should equal in the transition state. At the same time, for the transition structure, there should be no oscillation between the atoms, so for the internuclear distance VS time curve, there should be a linear line e.g.Fig.2. Furthermore, for the saddle point (transition state), the momentum should equal 0 otherwise it will also create the oscillation in the potential energy surface and will not locate on the saddle point, eg.Fig.2.2. By changing the value of r and keeping momentum 0, we aim to have linear lines without oscillation in the 'Internuclear distance VS time' curve. (Fig.2) In the surface plot, there should be a single dot on the surface plot, like Fig.2.1.
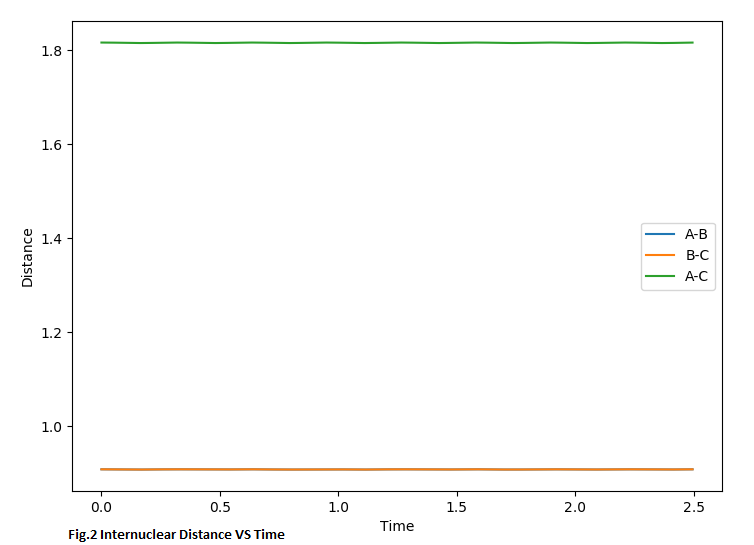
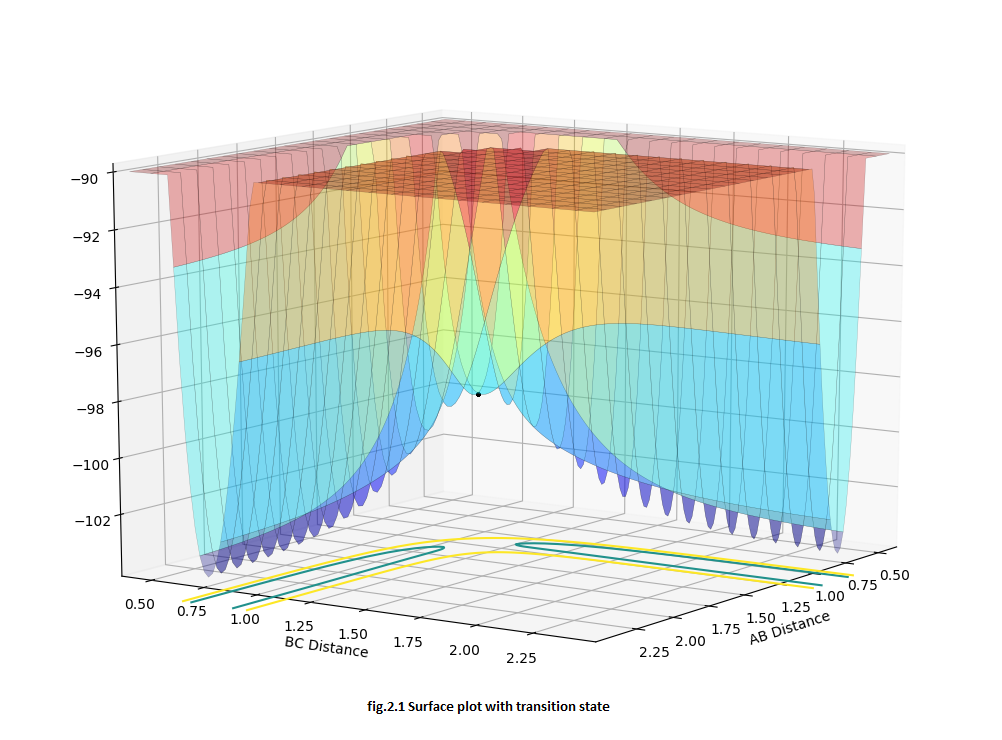
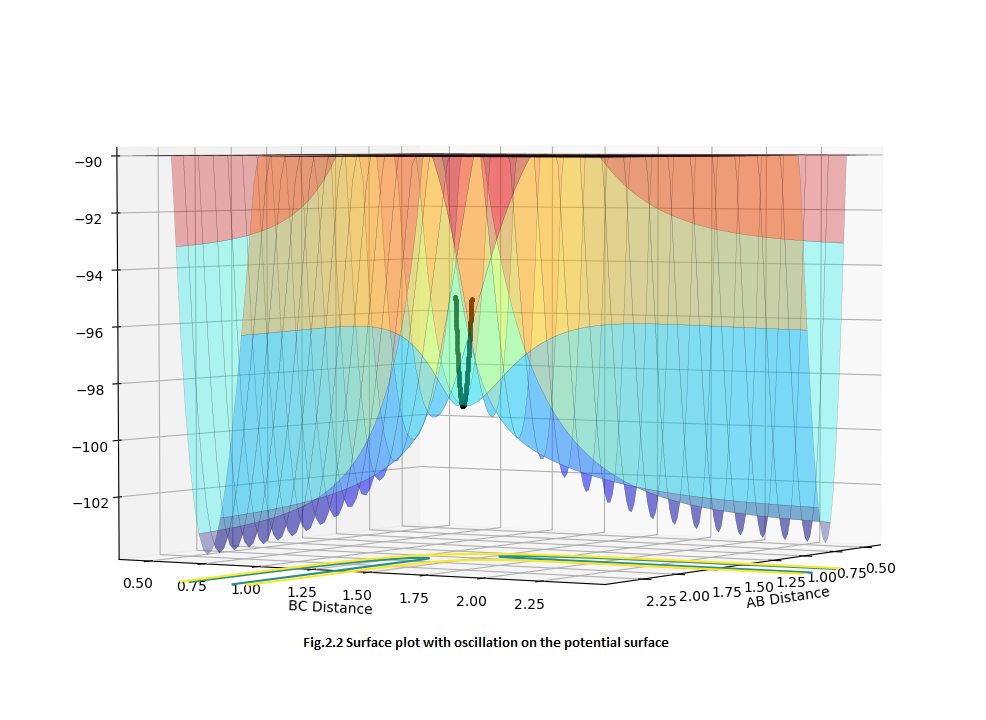
MEP and Dynamic calculation
3.Comment on how the mep and the trajectory you just calculated differ.
For the mep calculation, there is no oscillation in the surface plot. This is because the mep calculation just tries to find the minimum point of the energy. If we treat the dynamic calculation as the real reaction trajectories, the mep is only one of the possible trajectory with the infinitely slow motion. Basically, the calculation mode is just to calculate the point of the energy curve with 0 gradients. By using the mep calculation, we can know which side the point is on the system.
There is no oscillation in the mep calculated contour plot (Fig.3.1) since the mep calculation only deals with the lowest energy pathway by simply calculating the derivatives.

But for the dynamic calculation, the oscillation was shown in the contour plot (Fig.3.2) since the dynamic calculation also takes account of the diatomic vibration.
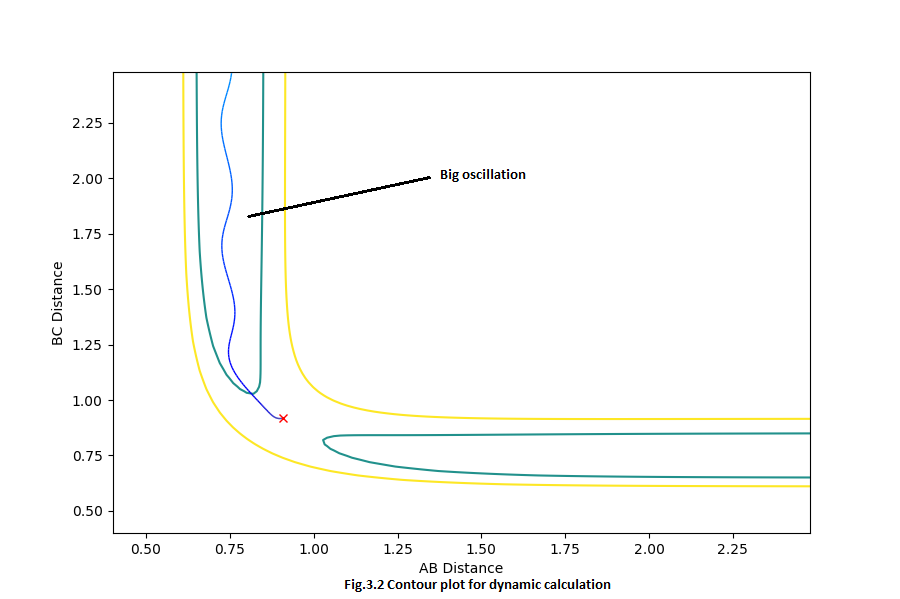
For the Internuclear distance VS time curve, when you increase the steps to a really large value, finally you will have the same value of internuclear distance. Since for dynamic calculation, one step will correspond one point in the trajectory line, but for mep calculation, maybe 10 steps was needed to get one point in the trajectory line. That is the reason why for the same number of step, there are different values of internuclear distance. Furthermore, the mep is a very special trajectory that corresponds to the infinitely slow motion, thus there will be zero momentum in the Internuclear momentum VS time curve. But for the dynamic calculation, the oscillation was also taken into account, thus an associated momentum will be shown in the curve.
Jas213 (talk) 11:02, 28 May 2018 (BST) "By using the mep calculation, we can know which side the point is on the system."I know what you are trying to say, but not very good scientific language.
Reactive and Unreactive trajectories
4.Complete the table by adding a column with the total energy, and another column reporting if the trajectory is reactive or unreactive. For each set of initial conditions, provide a plot of the trajectory and a small description of what happens along the trajectory.
| p1 | p2 | total energy (kcal/mol) | Reactive or not |
|---|---|---|---|
| -1.25 | -2.5 | -99.018 | reactive |
| -1.5 | -2.0 | -100.455 | unreactive |
| -1.5 | -2.5 | -98.955 | reactive |
| -2.5 | -5.0 | -84.954 | unreactive |
| -2.5 | -5.2 | -83.414 | reactive |
By using p1=-1.25 and p2=-2.5, the total energy is -99.018 kcal/mol and is a reactive pathway. The trajectory from reactant to transition state is quite smooth with small oscillation due to the small momentum value used. After the transition state, the oscillation is much bigger due to the extra momentum is given by crossing the transition state. Before entering the transition state, the H-H bond starts to break and dissociate, but the incoming H and the other H atom from H2 molecule start to form a bond. When they pass the transition state, the H2 molecules was dissociating and a new H-H bond was formed.
By using p1=-1.5 and p2=-2.0, the total energy is -100.455 kcal/mol and is an unreactive pathway. Under this combination of momentum, the trajectory cannot reach the transition state The reason may be the lack of enough kinetic energy to overcome the energy barrier/activation energy.
Jas213 (talk) 11:14, 28 May 2018 (BST) "the lack of enough kinetic energy" is not very accurate. P1 has even more kinetic energy than in the example before. What do p1 and p2 represent? If you say it's due to different amounts of kinetic energy, it would be good to have stated them as well and not just the total energies.

By using p1=-1.5 and p2=-2.5, the total energy is -98.955 kcal/mol and is a reactive pathway. By increasing the value of p2 momentum, the pathway switch from unreactive to reactive which can be attributed to the increasing kinetic energy. After passing the transition state, a product-like molecule start to form and the reactants will dissociate.
By using p1=-2.5 and p2=-5.0, the total energy is -84.954 kcal/mol an is an unreactive pathway. Since the momentum value is quite high for this condition, after crossing the barrier the oscillation is very big but it is still an unreactive pathway. The trajectory has already passed the barrier and move towards the product channel, but it recrosses it and goes back to the reactant again which means that this momentum value does not allow the trajectory enter the product channel although it can cross the state.
By using p1=-2.5 and p2=-5.2, the total energy is -83.414 kcal/mol and is a reactive pathway. From the surface plot, the trajectory passes through the barrier and go back and then recross it again, then it moves towards the product channel. This process basically shows that even the bond was formed during the transition state, it may still have some possibilities to go back. After entering the product channel, it starts to have a big oscillation which means the quick exchange between potential and kinetic energy.
Jas213 (talk) 11:17, 28 May 2018 (BST) What did you learn from this experiment/table? An overall concluding comment would have been expected.
Assumption of Transition state
5.State what are the main assumptions of Transition State Theory. Given the results you have obtained, how will Transition State Theory predictions for reaction rate values compare with experimental values?
Assumption:
1.the atoms in the reactant state have energies that are Boltzmann distributed, so it means that the system needs to have enough time to become thermally equilibrated to satisfy this assumption.
2.when the system pass through the transition state with the velocity towards the product channel, the system will not go back to the reactant.
3. the quantum tunnelling effect was negligible.
4. the Born-Oppenheimer approximation is invoked
For the quantum tunnelling effect, since the low energy molecules still have the possibilities to tunnel through the energy barrier, so the actual rate may be higher than the predicted rate. But since the tunnelling effect depends on the mass of the atom, so it may have an effect on the light molecules, but for the heavier molecules, the effect is negligible.
Since for the transition state theory, once the system attains the transition state, it will not go back to the initial state again. But the reaction trajectory may pass through the transition state with a much higher energy, so it will recross the barrier back towards the reactants, so for the actual experiment, the system may still have the possibilities to go back towards the initial state, thus this may decrease the rate. As a result, this assumption may cause the experimental rate may be lower than the predicted rate.
Jas213 (talk) 11:18, 28 May 2018 (BST) Do you know your TS theory by heart? Where are your references? Where have you taken the assumptions from?
H-F + H system & H-H + F system
PES Inspection
6.Classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved?

For F + H2 reaction, it is an exothermic process since the product energy level is much lower than the reactant energy level which was shown in Fig.5.1. Since it is an exothermic reaction, the energy absorbed for bond breaking is smaller than the energy released for bond formation. So it means that the broken bond is weaker than the formed bond. As a result, the bond between H2 is weaker than H-F bond.
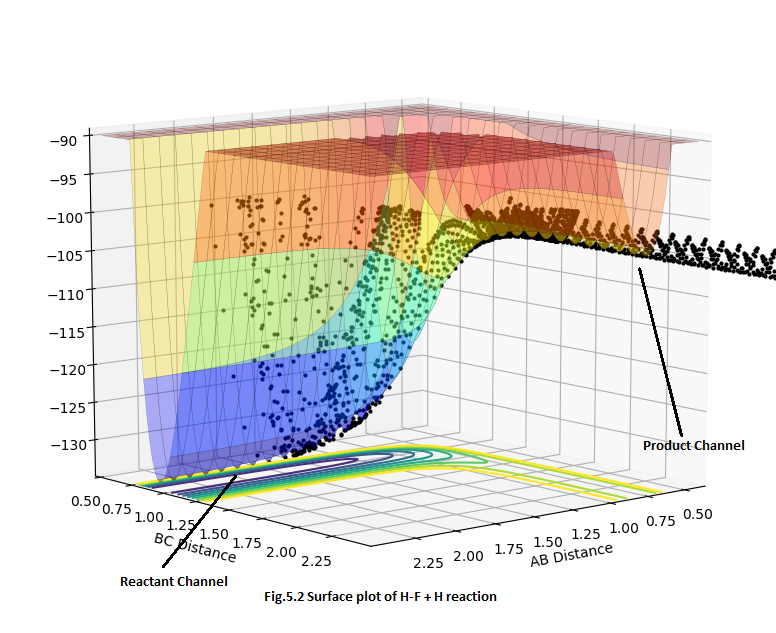
For H + HF reaction, it is an endothermic process since the product energy level is much higher than the reactant energy level which was shown in Fig.5.2 Since it is an endothermic reaction, the energy absorbed for bond breaking is bigger than the energy released for bond formation. So it means that the broken bond is stronger than the formed bond. As a result, the bond between H-F is stronger than H2 bond.
Jas213 (talk) 13:03, 28 May 2018 (BST) Why is the HF bond stronger than the HH bond? How could you have illustrated this with some numbers?
Transition state coordination
7.Locate the approximate position of the transition state.
The approximate position of the transition state for F + H2 reaction is:
rAB=1.8087 Å
rBC=0.7499 Å
The approximate position of the transition state for H + HF reaction is:
rAB=0.745 Å
rBC=1.8107 Å
Jas213 (talk) 13:06, 28 May 2018 (BST) What method did you use? How did you find the TS? How do you know it is a TS? 8.Report the activation energy for both reactions.
Activation energy calculation of F + H2 reaction:
potential energy of reactant = -104.020 kcal/mol
potential energy of transition state = -103.742 kcal/mol
activation energy = 0.278 kcal/mol
Activation energy calculation of H + HF reaction:
potential energy of reactant = -133.957 kcal/mol
potential energy of transition state = -103.752 kcal/mol
activation energy = 30.205 kcal/mol
Again what method did you use? Where are your meps ?
Reaction dynamics
9.In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. How could this be confirmed experimentally?
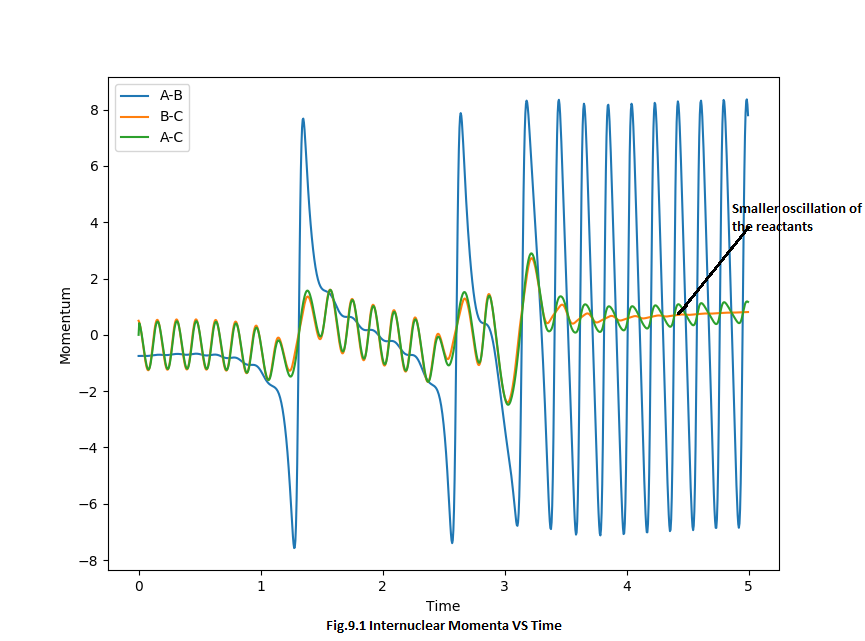
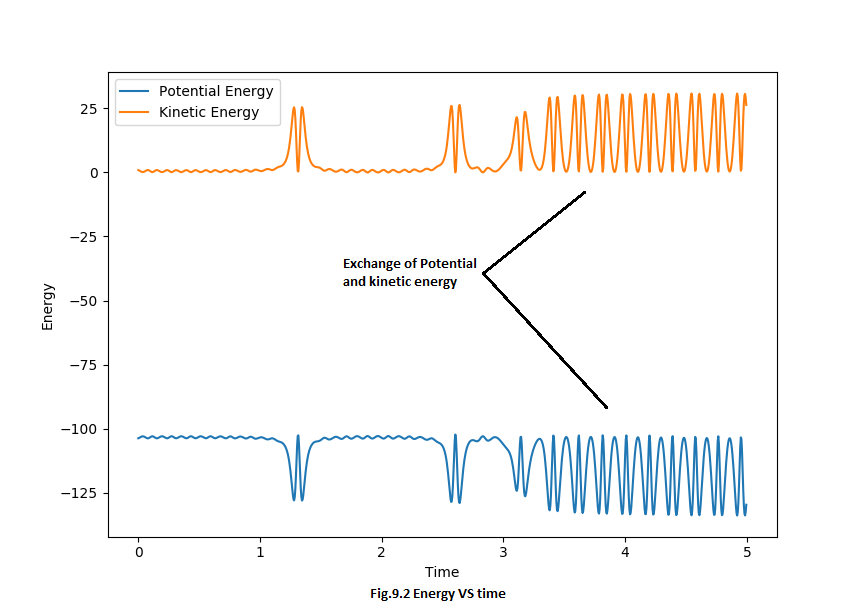
Since the energy is conserved, the reaction energy was released as vibrational kinetic energy. When reactants pass through the transition state, the H2 molecule will have a smaller momentum and kinetic energy which can be shown on the Internuclear momentum VS time graph, Fig.9.1. But for the product-like molecule, it starts to gain additional momentum and bigger vibrational kinetic energy. This also can be proved by the Energy VS time curve (Fig.9.2), on average, the kinetic energy increase from roughly 0 kcal/mol into 17 kcal/mol which indicates the exchange between potential and kinetic energy. Also from the animation, the oscillation of the product-like molecule oscillate much more stronger than the reactant-like molecule which represents the stronger vibrational kinetic energy.
By using IR spectrometer, the vibrational frequency of product and reactants can be obtained. If the vibrational frequency corresponds to the product is much higher than the reactants, it can demonstrate that the potential energy was transformed into vibrational kinetic energy.
By measure the heat transfer by calorimetry, we can measure the amount increased on kinetic energy. Since vibrational energy also belongs to the kinetic energy, by measuring the heat transfer, the heat released can also demonstrate the change of the potential energy into the kinetic energy. This can be used as extra evidence under some assumptions.
10.Setup a calculation starting on the side of the reactants of F + H2, at the bottom of the well rHH = 0.74 Å, with a momentum pFH = -0.5, and explore several values of pHH in the range -3 to 3 (explore values also close to these limits). What do you observe? Note that we are putting a significant amount of energy (much more than the activation energy) into the system on the H - H vibration.
As I keeping changing the value of momentum of pHH, some of the reactions are reactive, but some of the reactions are unreactive although the energy we put is much more than the activation energy.
For example, when the pHH equals -2.5 and other conditions are kept constant, it is an unreactive pathway. But when I change the pHH into -2, it switches into a reactive pathway. Then, a slight change on pHH = 0.7 will change it into an unreactive pathway.
Jas213 (talk) 13:09, 28 May 2018 (BST)Where are your plots of the trajectories? It started off like a very strong report. It is a shame that you didn't show the same effort for exercise 2.
11.For the same initial position, increase slightly the momentum pFH = -0.8, and considerably reduce the overall energy of the system by reducing the momentum pHH = 0.1. What do you observe now?
Although the reaction has smaller energy, the reaction is still a reactive pathway. The energy is -103.364 kcal/mol, but for the system with pFH=-0.8, pHH=-2.5, the energy is -98.659 kcal/mol. Compare this 2 values, the system with lower energy is a reactive pathway, but the system has higher energy is an unreactive pathway.
12.Discuss how the distribution of energy between different modes (translation and vibration) affect the efficiency of the reaction, and how this is influenced by the position of the transition state.
The position of transition state can greatly affect the reaction pathway. For the reaction with early transition state, translational energy is very effective in overcoming the energy barrier, since the translational energy allow the trajectory pass through the transition state more directly, but for the vibrational energy, the trajectory will vibrate and hard to pass through the transition state, sometimes, it may recross the transition state and go back to the reactants again.
For the reaction with late transition state, translational energy is less effective to allow the trajectory passes the transition state since the translational energy is not efficient to allow the trajectory to cross the turning point (late transition state). But for the vibrational energy, it will force the trajectory to oscillate and pass through the turning point more easily. As a result, for a reaction to take place, the reaction not only need to have a sufficient energy to overcome the energy barrier but also need to have a correct combination of vibrational mode and translational mode at right time. If the initial conditions were changed slightly, this may cause the reaction switch from a reactive pathway into an unreactive pathway.
Jas213 (talk) 13:14, 28 May 2018 (BST) Here you were expected to mention Polanyi's rules and give references.