MRD:01567335
Missing questions, no references given. Engagement with the experiment is very minimal, with only the results being presented briefly in most cases. Effectively, I would consider 2-3 questions to be answered acceptably. Fdp18 (talk) 15:14, 24 May 2020 (BST)
Exercise 1
On a potential energy surface diagram, how is the transition state mathematically defined? How can the transition state be identified, and how can it be distinguished from a local minimum of the potential energy surface?
This answer is much too brief, and the first part is incorrect. The transition state is not a maximum. I strongly recommend you to revise the underlying theory, starting with curve sketching. Maybe also have a look at the first year gaussian labs. Make sure you understand the following: Stationary point, local and global extrema, minimum and maximum, saddle point before you move on to Transition States. Also bear in mind that any chemical system with more than 2 atoms has many internal coordinates which span the PES - for Methane, the PES is already a function in 3*5-6=9 dimensions! Fdp18 (talk) 14:48, 24 May 2020 (BST)
The transition state is located at the maximum on the potential energy surface. At this point, the gradient of the potential(∂V(ri)/∂ri) is zero.
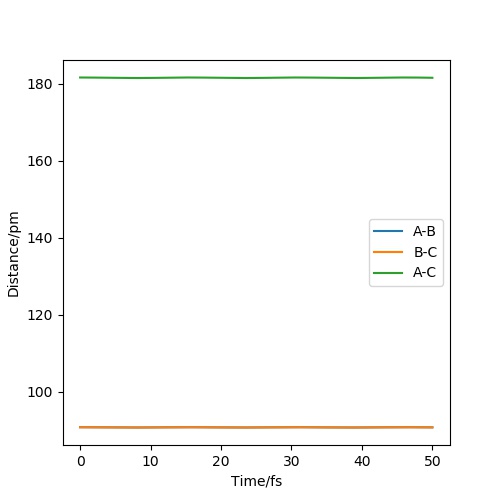
Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” plot for a relevant trajectory
The transition state position (rts) is at 90.8 pm. It can be seen in the image that if the initial r1 and r2 are set at 90.8 pm, the distance remains constant. This means the system is at the transition state since it doesn't undergo any periodic vibration.
This answer is too brief. It is not possible to reproduce the results with the information given - the result might be correct, but it is not obvious how you arrived there. I cannot tell if you actually did the experiment yourself or just took the final value of someone else and produced the picture. For future reference: show us what you did! It is like with a synthetic experiment: it's not enough to just hand in a few grams of the pure product, because you could have just bought it. You need to show some form of engagement with the experiment this is more important than just reporting the exact right value. Fdp18 (talk) 14:53, 24 May 2020 (BST)
Comment on how the mep and the trajectory you just calculated differ
On the dynamics trajectory, it is seen that after the molecule AB is formed, it undergoes vibrational motion as it moves away from C. However, this cannot be seen in the mep trajectory
Same as above, no demonstration of engagement. Fdp18 (talk) 14:53, 24 May 2020 (BST)
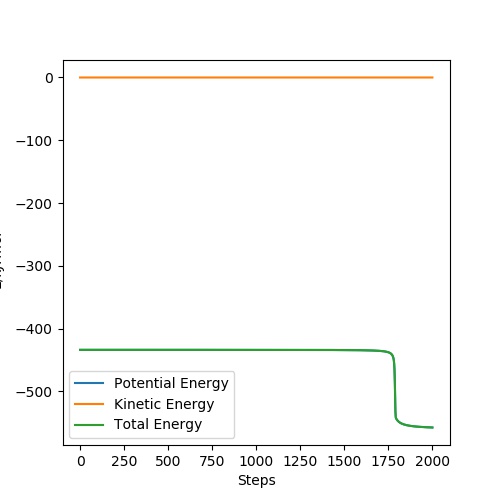
Complete the table above by adding the total energy, whether the trajectory is reactive or unreactive, and provide a plot of the trajectory and a small description for what happens along the trajectory. What can you conclude from the table?
| p1/ g.mol-1.pm.fs-1 | p2/ g.mol-1.pm.fs-1 | Etot | Reactive? | Description of the dynamics | Illustration of the trajectory |
|---|---|---|---|---|---|
| -2.56 | -5.1 | -414.28 | yes | A collides with BC and forms the molecule AB. C then moves away from AB | 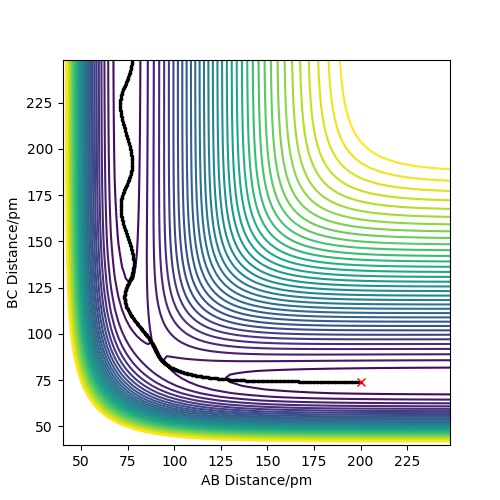
|
| -3.1 | -4.1 | -420.077 | no | A moves towards BC, but doesnt collide with it. Think about what 'colliding' means - it doesn't need to be reactive to collide. The Nitrogen molecules in air collide all the time, but they don't react with each other. Fdp18 (talk) 14:56, 24 May 2020 (BST)
It then moves away from BC ||
| |
| -3.1 | -5.1 | -413.977 | yes | A collides with BC and forms the molecule AB. C then moves away from AB | 
|
| -5.1 | -10.1 | -357.277 | no | A collides with BC and forms the molecule AB. C moves away from AB, and then moves back towards AB. C collides with AB and forms BC. A then moves away from BC. | 
|
| -5.1 | -10.6 | -349.477 | yes | A collides with BC and forms the molecule AB. C moves away from AB, and then moves back towards AB. C collides with AB and forms BC. A moves away from BC and then moves back toward BC. A collides with BC and forms AB. C then moves away from AB. | 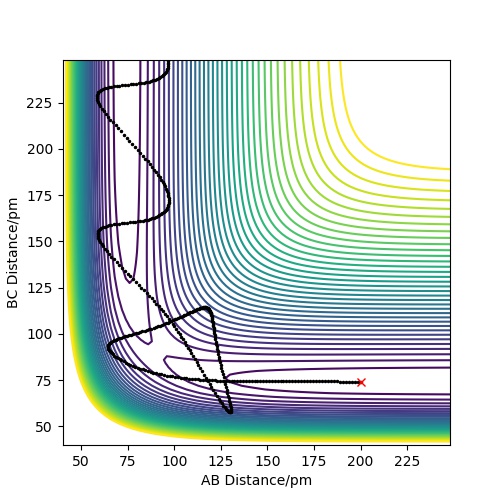
|
The description is correct and complete - however, there is no conclusion. The aim of this degree is to learn to think for yourself, not to merely produce data. Think about the bigger picture - if you look at all the entries in this table, what can you conclude from it? do you learn something about reaction rates or reaction trajectories in general? Fdp18 (talk) 14:58, 24 May 2020 (BST)
--> Missing a question here Fdp18 (talk) 15:01, 24 May 2020 (BST)
Exercise 2
By inspecting the potential energy surfaces, classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved?
In the potential energy surface of F + H2, it is seen that the energy of the reactant is higher than that of the product. Hence, it is an exothermic reaction. The tranisition state is closer in energy to the reactant. The reverse reaction, H + HF, is an endothermic reaction. The bond strength of HF is greater than that of HH. Hence, HF is at a lower energy than HH and more energy is required to break its bond.
This part is also brief, but it contains all required information. Is "The bond strength of HF is greater than that of HH" a statement or a conclusion? You use it like the first, but there are no references given as to where you took your bond strengths from. Fdp18 (talk) 15:04, 24 May 2020 (BST)
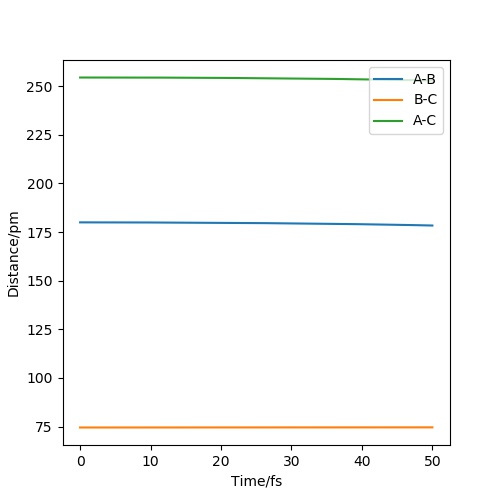
Locate the approximate position of the transition state
At the transition state, rab = 180pm and rbc = 74.5pm. In the figure, AB is FH and BC is HH.
Report the activation energy for both reactions
Activation energy of F + H2 = 106.016 kj/mol. Energy of the reactant is -433.984 kj/mol. Energy of the transition state is -540 kj/mol
Activation energy off by two orders of magnitude. Fdp18 (talk) 15:08, 24 May 2020 (BST)
There is a serious issue with understanding here - your transition state is lower in energy than your reactants. See also your figure - you took the energy of the products and then just changed the sign of the activation energy to be positive. Fdp18 (talk) 15:08, 24 May 2020 (BST)
Activation energy of H + HF = 398.201 kj/mol. Energy of the reactant is -141.799 kj/mol. Energy of the transition state is -433.984 kj/mol
Similar here, activation energy is quantitatively wrong. Fdp18 (talk) 15:08, 24 May 2020 (BST)
In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. Explain how this could be confirmed experimentally.
The F atom collides with the H2 molecule and forms the HF molecule. When the HF is formed, it gains kinetic energy. This energy results in the molecule moving from the molecular ground state to a vibrational excited state. The molecule then moves back to the ground state. Some of the energy is emitted in the form of radiation, resulting in the lowering of the total energy. This can be confirmed using IR spectroscopy. The intensity of the peak in the absorbtion spectrum will be lower than the intensity of the peak in the emission spectrum.
This part is ok. Fdp18 (talk) 15:09, 24 May 2020 (BST)
Discuss how the distribution of energy between different modes (translation and vibration) affect the efficiency of the reaction, and how this is influenced by the position of the transition state.
Higher vibrational energy leads to more efficient endothermic reaction. THis is beacuase if there was a high translational energy, the atom would collide with the molecule and then return in the opposite direction. Also, the direction of the vibrational motion aligns with the direction that fvours the formation of the product. Higher translational energy leads to a more efficient exothermic reaction. This is because higher vibration makes it harder to cross the barrier since the product is at a lower energy. The system will move up the vallet and form the reactants once again.
Brief, not supported with simulation results, reasoning is not clear. Fdp18 (talk) 15:11, 24 May 2020 (BST)