MRD:01503930yq618
Introduction
This lab studies the trajectories and relative behaviour of triatomic systems by simulation via lepsgui.py and looks into the importance of right energy distribution. H-H-H and F-H-H systems have been investigated specifically.
EXERCISE 1: H + H2 system
Dynamics from the transition state region
In a potential energy surface, the transition state is the first-order saddle point where it is a minimum along all coordinates except oneIt's on one coordinate, which is both a minimum and a maximum depending on the direction in which you measure. Pu12 (talk) 00:52, 27 June 2020 (BST) . To distinguish it from a local minimum, the hessian matrix could be found where the eigenvalues should be one positive and one negative. In contrast, the eigenvalues of a local minimum would be both positive and the second derivative would be positive. (see fig.1 below) Formula? Pu12 (talk) 00:52, 27 June 2020 (BST)
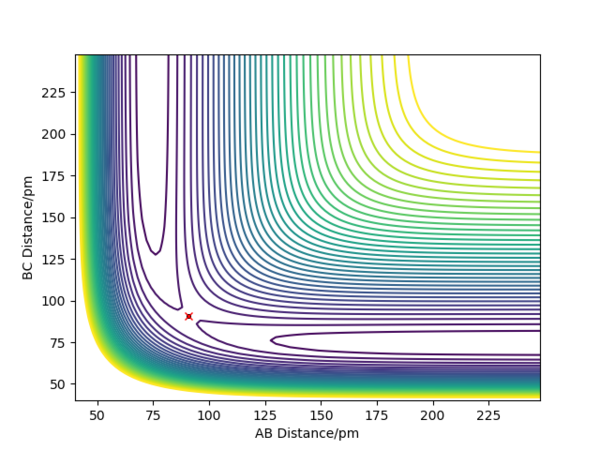
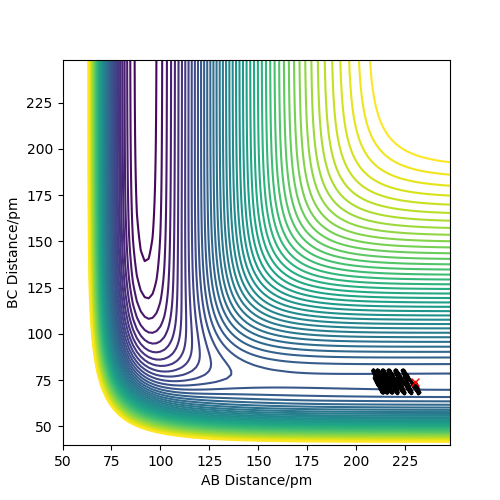
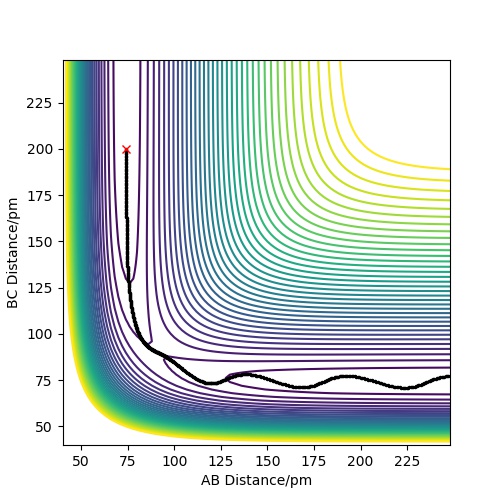
An estimate for the transition state position is rts=90.8pm. From the "Internuclear Distance VS Time" plot(see fig.2), it can be seen that rAB is equal to rBC in this case and the distance is constant, which means the reaction is now at transition state.
Calculating the reaction path
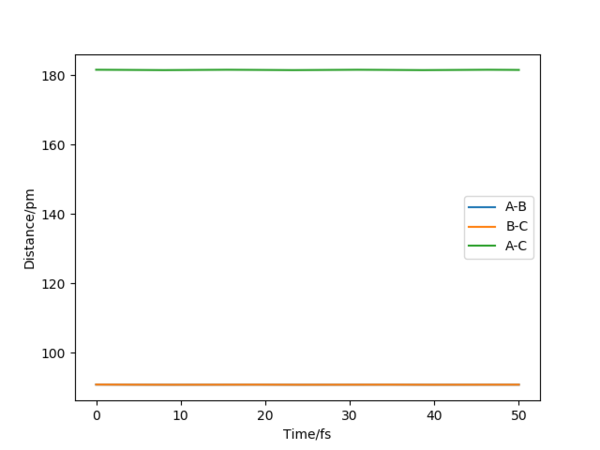
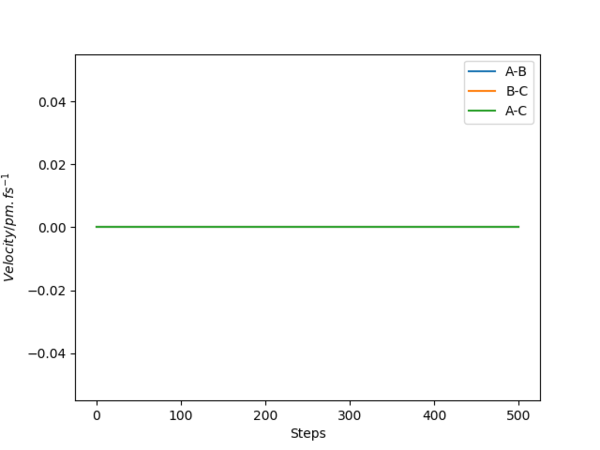
The minimum energy path corresponds to infinitely slow motion around the transition state and the momenta are set to zero, so the internuclear velocity of A-B, A-C and B-C remained zero for the mep trajectory(see fig.3), whereas in dynamics trajectory the velocities start to oscillate in a fixed frequency after reaching the transition state. Besides, the intermolecular distance in mep calculation remains unchanged for the newly formed H2 molecule which means it is not oscillating(fig.4).Good, but a surface plot showing the path would be more appropriate here. Pu12 (talk) 00:52, 27 June 2020 (BST)

Reactive and unreactive trajectories
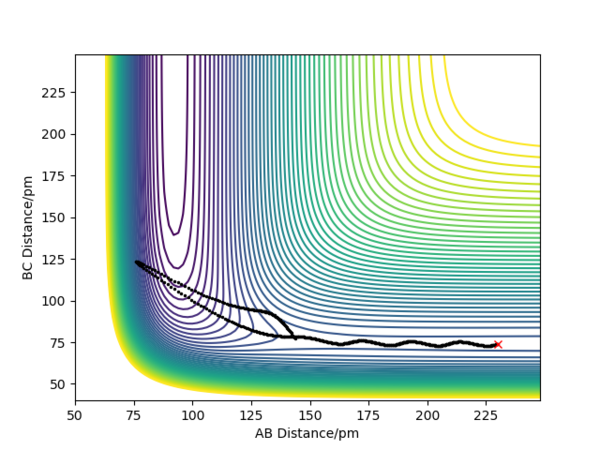
With the initial positions being r1 = 74 pm and r2 = 200 pm, a set of different momenta were used to run the trajectories (see table below). The results in the table emphasise the importance of the right energy distribution into the vibrational and translational mode and indicate the fact that just having high initial energy is not enough for the reaction to take place because of the possibility that the reactants may be reformed by recrossing the barrier.
| p1/ g.mol-1.pm.fs-1 | p2/ g.mol-1.pm.fs-1 | Etot/kJ.mol-1 | Reactive? | Description of the dynamics | Illustration of the trajectory |
|---|---|---|---|---|---|
| -2.56 | -5.1 | -414.280 | Yes | As H(A) approaches the bonded Hs, it forms a bond with H(B) and the original B-C bond breaks. Then the bonded A-B hydrogen molecule starts to vibrate and moves back together. | see fig.5 |
| -3.1 | -4.1 | -420.077 | No | H(A) is trying to approach the other two bonded Hs until it is stopped at some point and not able to form the bond as it is not reactive enough. Then H(A) moves back again alone. | see fig.6 |
| -3.1 | -5.1 | -413.977 | Yes | H(A) is approaching the two bonded Hs until the reaction happens and it forms a bond with H(B). Then the newly formed H2 molecule moves back together and vibrates. | see fig.7 |
| -5.1 | -10.1 | -357.277 | No | As H(A) approaches the bonded Hs, it forms a bond with H(B) and the bond starts to vibrate. But then the bond breaks, and H(B) moves back to bond with H(C) again. | see fig.8 |
| -5.1 | -10.6 | -349.477 | Yes | As H(A) is approaching the two bonded Hs, it first collides with H(B) but then H(B) bounces back before it collides with H(A) again to form a new A-B bond. After that, the A-B hydrogen molecule moves back together and vibrates. | see fig.9 |
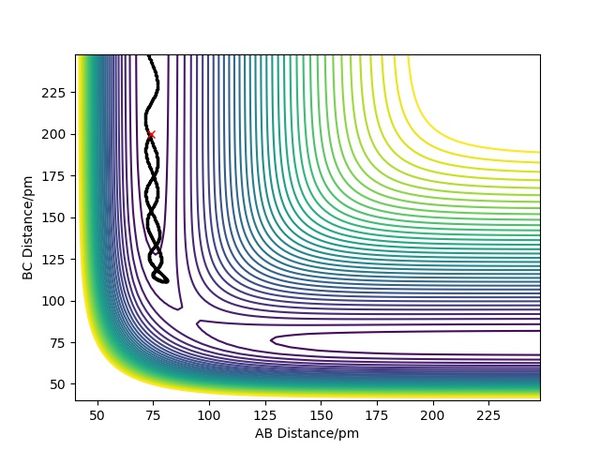
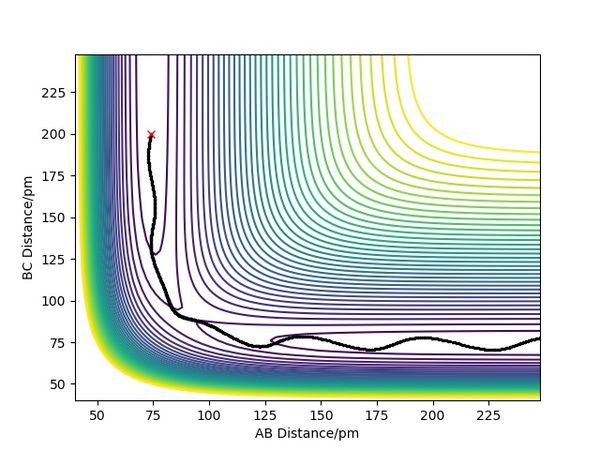
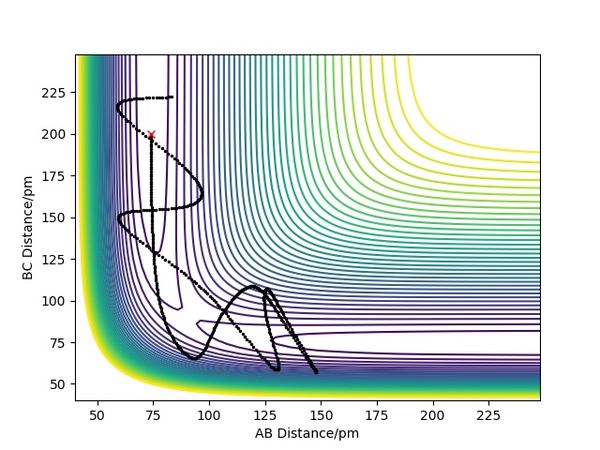
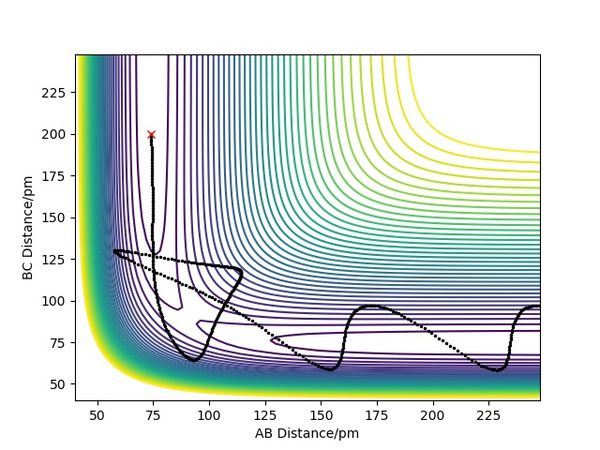
Overall summary? Pu12 (talk) 00:52, 27 June 2020 (BST)
Limitations of the transition state theory and the rate of reaction
In this case the transition state theory does not take into account the possibility that quantum tunnelling happens and the reactants can cross the energy barrier even without sufficient energy to form new bonds and become products, under which circumstance the reaction rate would be underestimated if using the theory only. This phenomenon based on quantum mechanics becomes more significant when the reaction has small energy barriers. The theory also assumes that the energy distributions would be what is expected from Boltzmann distribution, which is not always the case with short-lived reactants. Moreover, the theory fails when the reactants possess such high initial kinetic energy that they may go back to reactants again once they pass the energy barrier, in which case the rate values would be underestimated again Unlike quantum tunneling, this would cause the theory to overestimate rate values. Pu12 (talk) 00:52, 27 June 2020 (BST) .
EXERCISE 2: F - H - H system
PES inspection
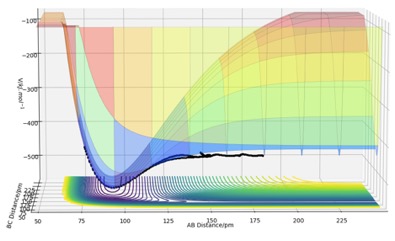
It can be seen from the surface plot of the F+H2 reaction that the energy of the products is smaller than that of the reactants, which means the F+H2 reaction is exothermic. On the other hand, Hammond's postulate states that the transition state of a reaction resembles the reactant or the product depending on to which it is closer in energy and in an exothermic reaction the transition state is closer to the reactant, which illustrates that the reaction is exothermic in this case. In terms of the relative bond strengths it indicates that the energy required to break the original bond is less than the energy released in forming the new bond, and in this case it means that the H-H bond is weaker than the H-F bond formed. When it comes to H+HF, the reaction is endothermic as the H2 bond formed is weaker than the HF bond.
Finding the transition state position
The transition state position is found to be r(HF)=181.1pm, r(HH)=74.5pm by trying different numbers until the relative motion is stationary. Plots like those used in the previous TS question and a discussion of how both the forward and reverse reactions have the same transition state would help this answer. Pu12 (talk) 00:52, 27 June 2020 (BST)
Finding the activation energy
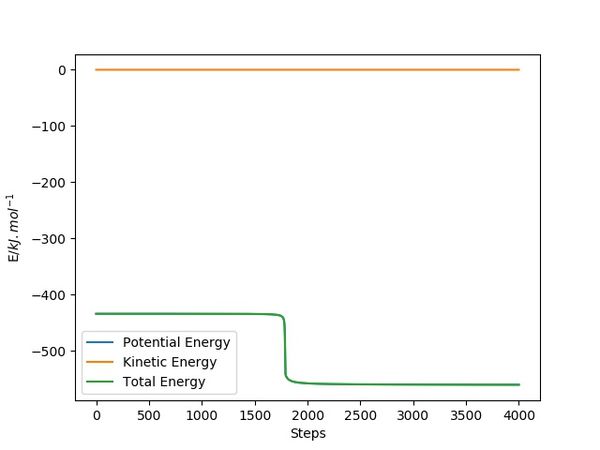
By changing the AB or BC distance by 1pm away from the transition state and setting the calculation type to MEP, an energy VS time plot of the H+HF reaction can be obtained as above. The activation energy is equal to the energy of transition state minus that of reactants, and from this we can calculate the energy difference from the plot which is 126.7kJ/mol. In the F+H2 reaction, the energy of the reactants is found to be -435.8kJ/mol, giving rise to the activation energy of 1.8kJ/mol.
Reaction dynamics and the release of reaction energy
As energy is conserved, the mechanism of the release of reaction energy has been suggested. When the F+H2 reaction happens, the reaction is exothermic and the potential energy is converted into vibrational energy. This could be confirmed using IR spectroscopy. When energy is released, the electrons are excited from the ground state to higher levels and the subsequent relaxation would give rise to two peaks shown in the IR spectrum. The intensity of the overtone would decrease whereas that of the fundamental would increase as time goes on. An energyThere would also be changes in translational energy which could be detected as temperature (not energy) by a calorimeter. Pu12 (talk) 00:52, 27 June 2020 (BST)
calorimeter could also be used.
Distribution of energies and the Polanyi's empirical rules
The Polanyi's rule states that the translational energy is better at promoting the reaction to overcome the barrier in reactions with early transition states, i.e. exothermic reactions. The translational energy helps promote exothermic reactions as it aligns with the direction that forms the products, and the trajectory would fall back into the lower energy state. However, the vibrational energy is in a different direction from the reaction path, which makes it harder for the trajectory to cross the barrier. By setting the initial conditions of rHH = 74 pm, rFH = 230pm, pFH = -1.0 g.mol-1.pm.fs-1 and exploring values of pHH in the range -6.1 to 6.1 g.mol-1.pm.fs-1, it can be seen that a lot of energy has been put into vibration and a high kinetic energy is required for the trajectory to be reactive. In contrast, when the exothermic reaction F+H2 happens and the transition state in early, a relatively low kinetic energy is required for the reaction to happen, which is in line with the Polanyi's empirical rule.Reference? Pu12 (talk) 00:52, 27 June 2020 (BST)