MRD:01503126
Exercise one: H + H2 system
On a potential energy surface diagram, how is the transition state mathematically defined?
δV(ri)/δri=0
This is also true for a minimum. You also need to comment about how the potential energy behaves in orthogonal directions with respect to the second derivative. Pu12 (talk) 20:07, 11 June 2020 (BST)
How can the transition state be identified ?
The transition state would be local maxima on minimum energy path that linking reactants and productsCorrect Pu12 (talk) 20:07, 11 June 2020 (BST) . When the transition state is reached, since force is the derivative of potential energy, The force would be zero.
and how can it be distinguished from a local minimum of the potential energy surface
A transition state is reached when the kinetic energy reaches its minimum Transition states and minima are properties of the potential energy surface, not of specific trajectories, and in that sense are unrelated to kinetic energy. João (talk) 10:37, 14 June 2020 (BST) , which means the first derivative of kinetic energy is zero and the second derivative of kinetic energy is greater than zero, also the potential energy is maximised: the first derivative of potential energy is zero and second derivative of potential energy is negative The second derivative of potential energy is negative along the reaction coordinate and positive along the orthogonal direction, this property is how the TS is distinguished from local minima. Pu12 (talk) 20:07, 11 June 2020 (BST) .
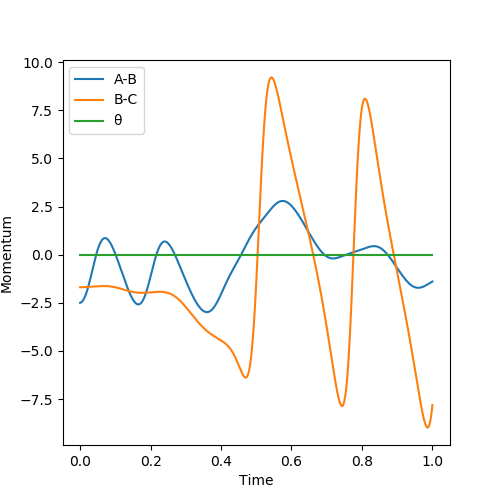
Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” plot for a relevant trajectory.
The best estimate of the transition state position exists when both the distances of AB and BC are 90.8 pm, the particles have zero momentum.
According to the Internuclear Distance vs Time plot, the distance remains unchanged, which corresponds to the zero kinetic energy and zero net interacting forces, otherwise correct Pu12 (talk) 20:07, 11 June 2020 (BST)
interacting forces between H2 molecule and H atom, so stationary nuclei at this specific distance, which is the saddle point.
Comment on how the mep and the trajectory you just calculated differ.
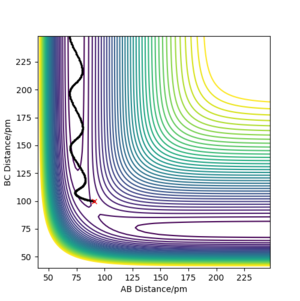
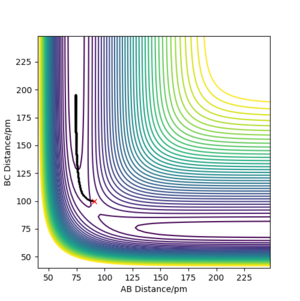
The difference between the two calculation types is whether the atomic motion is included or not. In mep the trajectory is a smooth line since it does not take atomic motion into account What do you mean? The B-C distance is increasing! João (talk) 10:37, 14 June 2020 (BST) . (as the right figure is shown below) In dynamic the trajectory is more like a curvature since it includes the atomic motion, it shows the oscillatory behaviour. (As the left figure is shown below)
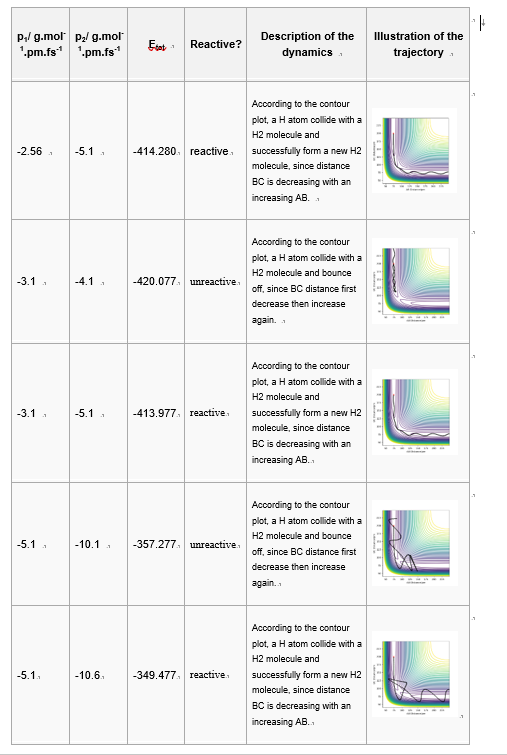
Complete the table above by adding the total energy, whether the trajectory is reactive or unreactive, and provide a plot of the trajectory and a small description for what happens along the trajectory. What can you conclude from the table?
You didn't comment on how vibrations change. Pu12 (talk) 20:07, 11 June 2020 (BST)
According to the results in the table, the hypothesis that all trajectories starting with the same positions but with higher values of momenta (higher kinetic energy) would be reactive, as they have enough kinetic energy to overcome the activation barrier is incorrect. For the 4th cases, p1 is -5.1 g.mol-1.pm.fs-1 and p2 is -10.1 g.mol-1.pm.fs-1, which both have greater kinetic energy to overcome the activation barrier, but the reactants do not form the transition state and reform the reactants again. Therefore enough kinetic energy of reactants does not ensure the reaction to take place.
Given the results you have obtained, how will Transition State Theory predictions for reaction rate values compare with experimental values?
The reaction rate predicted by Transition State Theory is overestimated comparing with the experimental value. The Boltzmann distribution has been assumed in the reaction so that the system has an evenly shared energy among the molecules, if the energy is unevenly distributed in the system, those thermally highly excited molecules would react differently than those thermally inactive molecules. But the transition state theory cannot predict that since not all the molecules are reacted at the same rate.
The transition state theory does not take quasi-equilibrium into account either, the reactants molecules can have lots of collisions, but some does not form the transition state and then break apart to reform the reactants again, these amount of products that does not pass through the barrier of activation energy does not have any effect between reactants and transition state equilibrium, which do not contribute to the equilibrium kinetics. What you are saying here is quite confusing. You seem to say that not all collisions are reactive, this is the case for all trajectories with total energy below the activation energy, so what is specifically wrong with transition state theory? You say in the above paragraph transition state theory estimates are an overestimate, but why? João (talk) 10:37, 14 June 2020 (BST)
Exercise two: F - H - H System
By inspecting the potential energy surfaces, classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved?
For F + H2, it's an exothermic reaction, which formed HF and H. So according to the FHH contour plot, the distance AB would decrease and distance BC would increase during the reaction, it's an exothermic reaction since the H-F bond formed is a stronger bond . (According to the figure of FHH system) You should discuss the energies that you get from the simulations, also a 3D suface plot would be more appropriate to demonstrate which reactant/product is at higher/lower energy level. Pu12 (talk) 20:07, 11 June 2020 (BST)
The reaction between H + HF is endothermic since the bond broken is weaker than the bond formed. (According to the figure of HHF system)

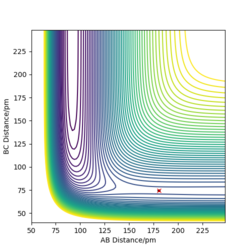
Locate the approximate position of the transition state.
For F + H2: p1= 180.6 pm, p2= 74.5 pm
For H + HF: p1= 74.5 pm, p2= 180.6 pm
You should include diagrams to show how you got these answers. Pu12 (talk) 20:07, 11 June 2020 (BST)
Report the activation energy for both reactions.
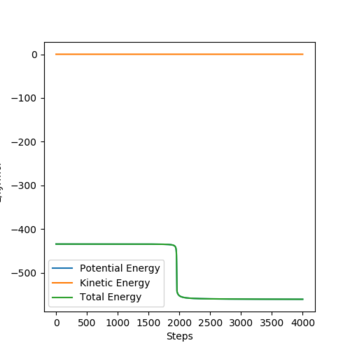
The activation energy is calculated from the difference between the energy of the saddle point (maximum energy of a reaction) and the energy of reactants. For F + H2 system, the maximum energy at the saddle point is -433.981 kJ/mol. In order to find the energy of reactants, the distance between F and H2 is set to a large value (10000 pm) to ensure the system has pure reactants state, then the energy of reactants could be found as -435.075 kJ/mol, so the activation energy for FHH system can be calculated to be 1.088 kJ/mol (dynamic).
For H + HF system. the maximum energy at the saddle point is -433.981 kJ/mol. To find the energy of reactants, the distance between H atom and HF molecule and the intramolecular distance in H2 are both decreases, then the lowest energy of reactants can be found from the Energy vs time graph as -560.256 kJ/mol. So the activation energy for HHF system can be calculated to be 126.275 kJ/mol.
Correct Pu12 (talk) 20:07, 11 June 2020 (BST)
In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. Explain how this could be confirmed experimentally.
The distance between atom F and H2 molecule and the intramolecular distance of H2 is set to be slightly different from the distance at the saddle point. Both momenta are set as zero therefore there is no kinetic force initially. According to the animation, the vibrating H2 molecule first approaches the atom F, as the distance between F and one of the H is close enough, a new H-F bond and a new H atom are formed. Then the HF molecule formed starts to vibrate and the H atom moves away from the HF. The newly formed HF contains some vibrational energy and the H atom gains some kinetic energy to move further from the HF, which is shown on the momentum vs time graph.
An IR spectrum can also be used to confirm the release of the reaction energy. If mainly the ground state of vibrational energy level is occupied, a single peak will appear on IR. If some particles occupy the 1st level of vibrational energy level, a second peak with lower overtone will appear at lower wavenumber.
If the energy is not conservedI'm not sure what you are trying to say here. Pu12 (talk) 20:07, 11 June 2020 (BST) , there would be heat loss during the release of the reaction energy, therefore the 2nd peak would have a lower overtone due to the heat loss.
Discuss how the distribution of energy between different modes (translation and vibration) affect the efficiency of the reaction, and how this is influenced by the position of the transition state.
Translational energy is better at promoting exothermic reaction, vibrational energy is better at promoting endothermic reaction, according to Polanyi's rules. [1]
As for an exothermic reaction, the energy barrier is usually located as an early barrier which means an earlier transition state appears, the translational energy is more effective to overcome the reaction barrier than the vibrational energy. The trajectory would be harder to cross the barrier in vibrational motion because the direction of motion is different from the direction of trajectory in MEP.
However, for an endothermic reaction (late barrier reaction), the structure of transition state is product-like and barrier is in late transition, the vibrational energy is more effective in promoting reaction rate since the vibrational motion is orthogonal to the energy path.
Did you observe anything like this in your simulations? João (talk) 10:37, 14 June 2020 (BST)