MRD:01493832
Molecular Reaction Dynamics: Applications to Triatomic systems
The transition state
On a potential energy surface diagram, the transition state is mathematically defined as a stationary point where the derivative of the potential energy curve, which is a force I'm not sure exactly what you mean here? Mak214 (talk) 18:17, 29 May 2020 (BST) , is zero. This means that there are no movement at the transition state.
The transition state is different from a local minimum of a potential energy surface in a sense that it is a minimum in one direction yet a maximum in another.1 Therefore, it is a stationary point but not a point of inflection. From the calculated forces, we are able to confirm that this is a stationary point and from the given corresponding eigenvalues/vectors, the positive and negative combination confirms that this is a saddle point. If we move away from the stationary point, the eigenvalues/vectors will be either all positive or all negative. (For a local minimum, the eigenvalues/vectors will be positive in all directions.) IOK. Mak214 (talk) 18:17, 29 May 2020 (BST)
H + H2 : Locating the transition state
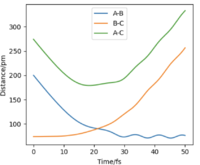
In the case of H + H2, AB and BC distances must be equal in the transition state because all atoms are identical so the transition state is symmetrical and its position can be identified by using a trial and error process, where the AB and BC distances in the initial conditions are varied until the corresponding forces become as close to zero as possible. For 3 Hydrogen atoms, AB and BC distances (rts) are found to be 90.775 pm.

From Fig.1, it is evidently clear that at rts = 90.775 pm, the distances are constant throughout confirming that it is a stationary point (i.e. the nuclei do not move) and that distances AB = BC. I Good. Well done. Mak214 (talk) 18:17, 29 May 2020 (BST)
Calculating the reaction path
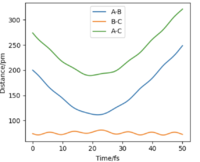
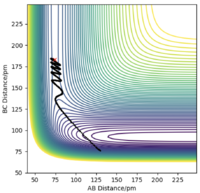
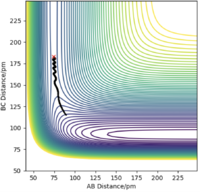
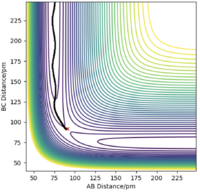
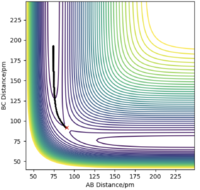
There are two types of calculation that can be done when considering the trajectory of the Hydrogen molecule: MEP (minimum energy path) and Dynamics. In MEP, atoms are assumed to be in an infinitely slow motion which corresponds to zero momenta at each time step. Good. Mak214 (talk) 18:20, 29 May 2020 (BST) Both calculations can be done by changing the initial conditions to r1 = rts+δ and r2=rts in order to visualise the trajectories. The difference between MEP and Dynamics are shown in two contour plots below. From the MEP contour plot, we can see that the black trajectory line is rather smooth as it approaches the transition state position marked with a red cross compared to the trajectory shown in Dynamics contour plot. The MEP calculated trajectory also starts off at a higher potential energy position than the one from Dynamics calculation. Hmm.. they should start with the same energy if you put in the same settings. Mak214 (talk) 18:20, 29 May 2020 (BST)
Because in MEP calculation, atoms are moving very slowly so the vibration between the reactant HA-HB is so small that it does not display an oscillating behaviour as in the Dynamics calculation. Not exactly, the MEP calculation there should be no vibration of the bond whatsoever since there is no momentum to move the atoms away from the equilibrium bond distance which is a valley in the potential energy surface. We only see vibration in the dynamics type calculation because there is residual momentum which allows the atoms in the molecule to vibrate. Mak214 (talk) 18:20, 29 May 2020 (BST)
 |
 |
Change in initial conditions
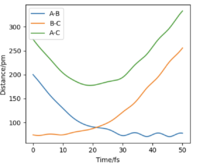
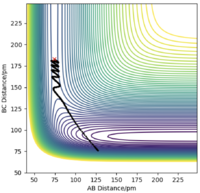
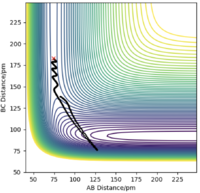
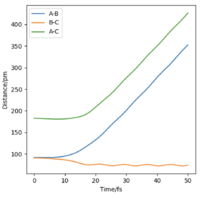
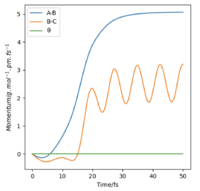
If we change the initial condition so that r1 = rts and r2=rts+δ instead, the result is still identical to previous result but with atom A moving away at large t and atom B bonding with C instead. By investigating the “Internuclear Distances vs Time” and “Momenta vs Time” plots and taking note of values for r1(t) r2(t) and p1(t) p2(t) at very large t (50 fs), another set of calculations can be done with using these values for the initial positions and momentum (with reversed sign). The observation is for “Internuclear Distances vs Time” plot, the result is a reflection in the y-axis of Fig. 4 meaning, the bonded BC H2 molecule and the previously isolated HA atom are now approaching each other and their positions at large t corresponds to the transition state position. With reversed sign of the momenta, this is a reverse process of what was previously done, instead of atoms moving apart, they are now moving towards each other with the same kinetic energy. The “Momenta vs Time” is now reflected in the x-axis of Fig. 5, which can be reasoned by the repulsion that the atoms experienced from coming together resulting in positive gradient which is gradual at first then steepens at high t as the three atoms are now very close.
 |
 |
Reactive and unreactive trajectories
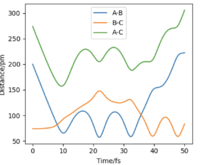
To test whether trajectories starting at the same position but with higher momenta will all react, a table has been constructed shown below, where r1=74 pm and r2=200 pm.
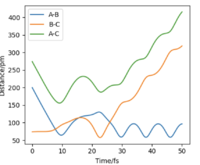
The last plot on the left corresponds to p1 = -5.1 g.mol-1.pm.fs-1, p2 = -10.6 g.mol-1.pm.fs-1 and Etot = -349 kJ.mol-1. This is a reactive trajectory since the approaching H forms a new bond then bounces back but did not break so overall, this trajectory is reactive.
- The last line of the table is not showing so I had to insert it like this.
Well done. It would be nice to have a line or two here concluding what you have learned from these trajectories. Mak214 (talk) 18:49, 29 May 2020 (BST)
Transition State Theory
The transition state theory is a classical consideration which assumes:2
1) The reactants and transition state are in a quasi-equilibrium. This means that once the reactants reaches the transition state, they cannot ? Mak214 (talk) 18:48, 29 May 2020 (BST)
collapse back to form the reactants again when in reality they can. This cause the values that the transition state theory predicts to be overestimated than the actual experimental value.
2) Quantum tunnelling is ignored. Because the reactants that overcome the barrier by moving across is ignored, this causes the values predicted by transition state theory to be underestimated than the actual experimental values.
3) All reactants that have enough energy to overcome the barrier will successfully form the product and the step that involves the formation of the product from the transition state is the rate-determing step. This causes an overestimation of the actual rate because in reality, although the reactants have high energy, but if the energy is not located in the right place (i.e. in the bond that needs to be broken) then the reaction will not happen. Good. Mak214 (talk) 18:48, 29 May 2020 (BST)
The F-H-H System
PES Inspection
F + H2
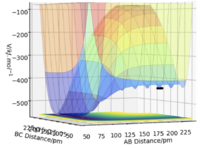
This is an unsymmetrical system which results in unsymmetrical potential wells in the surface plot.
From the surface plot, we are able to tell that this is an exothermic reaction. BC is the H-H distance of the reactant while AB is the H-F distance, the z-axis of the surface plot represents energy and in this case, the H-H is deeper in energy compared to H-F which corresponds to the energy profile of an exothermic reaction and thus, explains the greater H-F bond strength compared to H-H. HF is deeper (lower E) because it is the stronger bond.. Mak214 (talk) 19:06, 29 May 2020 (BST)
The approximate position of the transition state can be found by the same method that was used for three Hydrogen atoms: trial and error where the corresponding forces are as close to zero as possible. This method gives rF-HA = 180 pm and rHA-HB=74.5 pm. These positions are confirmed to be the transition state by the opposite signs of the eigenvalues/vectors. At this position, the energy of the transition state is -434 kJmol-1. Good. Mak214 (talk) 19:06, 29 May 2020 (BST)
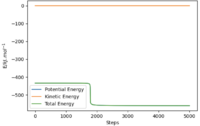
In order to find the activation energy, the position of the system is displaced slightly towards the reactant side from the transition state (rF-HA = 179 pm) and MEP calculation was used to obtain the "Energy vs Time" below.
From the graph, the energy of the reactants is -561 kJmol-1. The difference between the reactant state and the transition state energy is the activation energy which is the energy required to cross the barrier to form the product, in this case is +127 kJmol-1. Yes. Well done. Mak214 (talk) 19:06, 29 May 2020 (BST)
 |
 |
H + HF
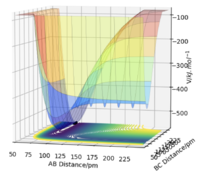
The surface plot of this reaction is a mirrored image of the above surface plot from the previous reaction. This shows that this is an endothermic reaction where the reactants have higher energy than the product. This correlates to the stronger H-F bond that requires input energy to break to form the H-H product. Same mistake as above, I think you were looking at this the wrong way around. The HH + F is the higher energy and the HF + H is the lower energy on the PES - reorient yourself with these axes so that this makes more sense. Mak214 (talk) 19:09, 29 May 2020 (BST)
The approximate transition state can be found in the same manner as the previous reaction and the positions are rF-HB = 180 pm and rHB-HC=74.5 pm, corresponding to total energy of -434 kJmol-1. The activation energy in this case is very small is reported to be +0.930 kJmol-1. Because of such small activation energy, it is easier to locate the position of the transition state by using the Hammond postulate. From the surface plot, we have deduced that this reaction is endothermic therefore it must have a late transition state meaning the structure of the transition state will resemble more of the product.3 As a result, shorter H-H distance and long F-H were predicted for the position of the transition state. Really good. Mak214 (talk) 19:09, 29 May 2020 (BST)
 |
 |
Reaction dynamics
One of the ways to confirm the mechanism of the release of reaction energy is by taking an Infrared absorption spectrum of the product. In this case, we can measure the IR spectrum of HF gas sample. When the reaction just happened and IR light is shown through, we are exciting the molecules that were once thermally relaxed and exclusively occupying the ground state to its first vibrational excited state and at early times (when the reaction just happened), we may also excitation from first to second vibrational excited state.
As a result, what we can observe in the IR spectrum is two peaks present, one is the fundamental peak and the other is an overtone appearing at a lower wavenumber. This is because of decreasing energy gap between each levels due to anharmonicity.4 By measuring the intensity of the overtone over time, we can extract the number of molecules that are vibrationally excited over time so we can expect the intensity of overtone to be smaller as energy is emitted as radiation and the intensity of the fundamental peak would increase as time goes on.
The other way to confirm the mechanism of energy release experimentally is by measuring the IR emitted directly, instead of probing the reaction using IR spectroscopy, as the molecules fall back from vibrational excited state to the ground state. This method can be done by using the Infrared Emission Spectroscopy (IES).5 Great. Mak214 (talk) 19:10, 29 May 2020 (BST)
F + H2
By setting up a calculation with rHH=74 pm, pFH=-1.0 g.mol-1.pm.fs-1 and pHH varied between -6.1 and 6.1 g.mol-1.pm.fs-1, it is visible from the contour plot that the trajectory only successfully rolled over to the product with pHH between -2.7 and 1.2 g.mol-1.pm.fs-1. Values outside of this range results in the trajectory hitting the wall and bouncing back.
H + HF
A reactive trajectory was obtained with a combination of pFH = 1 g.mol-1.pm.fs-1 and pHH = -7 g.mol-1.pm.fs-1. Decreasing the momentum of the incoming H further results in the trajectory hitting the wall and bouncing back while increasing the energy of H-F vibration causes the trajectory to go in the other direction rather than towards the product.
Polanyi's Empirical Rule
The above investigation illustrates the Polanyi's empirical rule. In an exothermic reaction, as demonstrated by F + H2, translational energy is favoured over vibrational energy because the trajectory can fall down into the low energy region of the PES whereas the vibrational energy is in a different direction and can cause the trajectory to bounce back near the transition state and cross back to the reactant state.
On the other hand, vibrational energy is favoured over translational energy in an endothermic reaction6 as illustrated by H + HF. This is because the trajectory has the same directionality with the vibrational energy that goes to the product. Nice report - see my comments. Mak214 (talk) 19:10, 29 May 2020 (BST)
Bibliography
1 Maxima, minima, and saddle points (article). https://www.khanacademy.org/math/multivariable-calculus/applications-of-multivariable-derivatives/optimizing-multivariable-functions/a/maximums-minimums-and-saddle-points (accessed May 22, 2020).
2 Peters, B. Transition State Theory. Reaction Rate Theory and Rare Events Simulations 2017, 227–271.
3 Libretexts. Hammond's Postulate. https://chem.libretexts.org/Bookshelves/Ancillary_Materials/Reference/Organic_Chemistry_Glossary/Hammond’s_Postulate (accessed May 22, 2020).
4 Libretexts. 13.5: Vibrational Overtones. https://chem.libretexts.org/Courses/Pacific_Union_College/Quantum_Chemistry/13:_Molecular_Spectroscopy/13.05:_Vibrational_Overtones (accessed May 22, 2020).
5 Keresztury, G. Emission Spectroscopy, Infrared. Encyclopedia of Analytical Chemistry 2006.
6 Zhang, Z.; Zhou, Y.; Zhang, D. H.; Czakó, G.; Bowman, J. M. Theoretical Study of the Validity of the Polanyi Rules for the Late-Barrier Cl CHD3 Reaction. The Journal of Physical Chemistry Letters 2012, 3 (23), 3416–3419.