MRD:01337001taw17
Molecular Reaction Dynamics: Triatomic Systems
Excercise 1: H + H2 System
Transition States
The transition state on a potential energy surface is mathematically defined as the point where , where V is defined as the potential energy of the system and q1 and q2 are orthogonal vectors where q1 is a tangent to the reaction pathway. At this point and . This is because the transition state is a saddle point. It represents the maximum energy point on the minimum energy pathway between reactants and products. Good.Sw2711 (talk) 16:15, 16 May 2019 (BST)The reactants will appear as local minimums along the reaction pathway. What about the products?Sw2711 (talk) 16:15, 16 May 2019 (BST)
Finding the Transition State
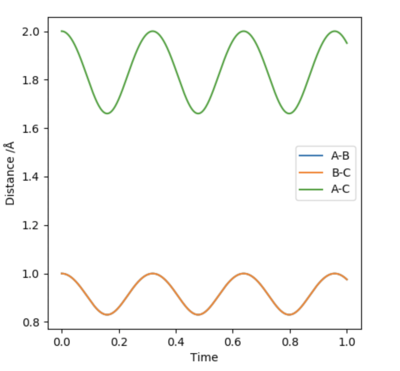
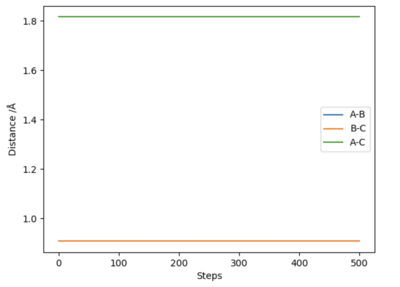

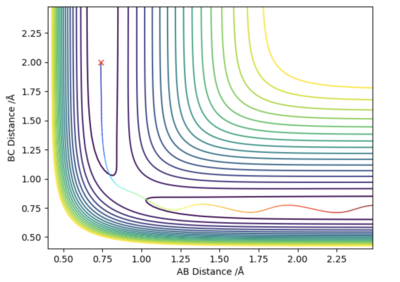
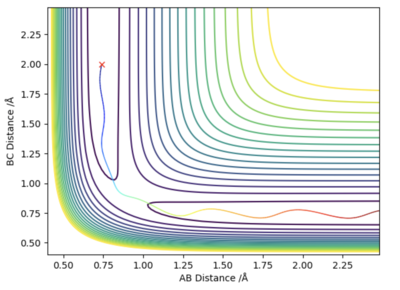
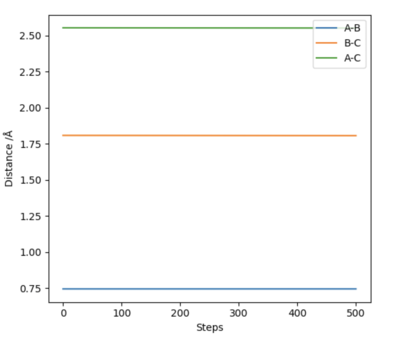
Since the system is symmetric, the transition state will occur when where is the distance between atom A and B, and is the distance between atom B and C. Using this condition the transition state can be located using an iterative method. A plot of internuclear distances against time can be generated as shown in figure 1. By finding the midway point between the height of the peaks and troughs a better estimate of internuclear distances in the transition state can be found. This is set as the new initial condition and the process is repeated. After three iterations, a transition state bond length, also known as rts of 0.908 was yielded. In the transition state for this system, the bond length will be a constant and will not oscillate. This is shown by the straight line in figure 2. Using an MEP method a very similar value of 0.9077 was generated.
Very good. You used two methods to validate your TS point! Well done!!Sw2711 (talk) 16:16, 16 May 2019 (BST)
MEP vs Dynamic Method
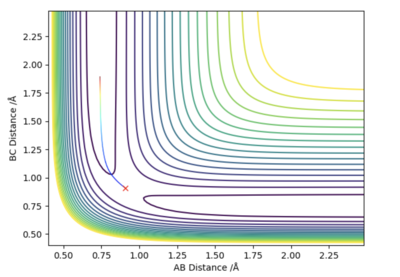
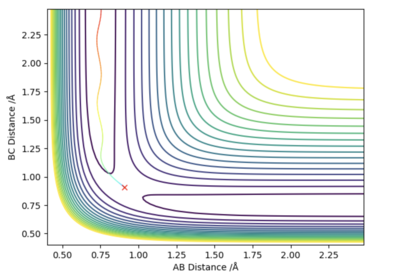
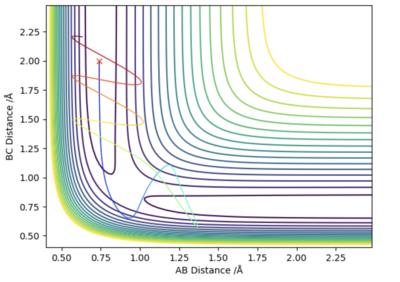
If one of the internuclear distances is set to rts + 0.01, the reaction will no longer remain in the transition state and instead will proceed to either the reactants or the products. A reaction pathway can then be plotted via two different approaches. As can be seen by figures 4 and 5, there are no oscillations in the minimum energy path (MEP) however there are in the trajectory predicted by the dynamic mode since it takes into account the vibrations of the molecule. The MEP is also much shorter than the path predicted by the dynamic mode. This is due to the fact that in the calculation of the MEP, momenta and velocities are reset to zero at each time step.
Good.Sw2711 (talk) 16:18, 16 May 2019 (BST)
Reactive and Unreactive Trajectories
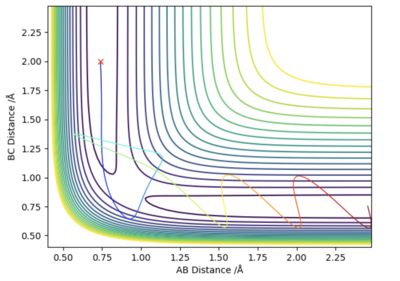
This part is good.Sw2711 (talk) 16:19, 16 May 2019 (BST) A series of scenarios were trialled to see under what conditions a successful reaction would occur. The fixed parameters used were: rAB = 0.74 and rBC = 2.0. Relative momenta between the atoms, pAB and pBC, were then changed.
From the table we can conclude that a successful reaction may not take place, despite the system having the required activation energy. As can be seen from the fourth scenario in the table, barrier recrossing is possible. It is therefore necessary that the system has the correct vibrational modes, as well as sufficient energy to cross the transition state, to ensure the products are formed.
Transition State Theory
Transition state theory, also known as activated complex theory, states that an activated complex is in equilibrium with the reactants and that the rate of product formation is dictated by the rate at which this activated complex passes through the transition state. (1) It also assumes the energy distribution of particles follows a Boltzmann distribution. (1) Despite transition state theory suggesting that reactant will not reform once the reaction pathway has gone through the transition state, as seen from scenario 4 above, this is not true. Experimentally, border recrossing is observed if the right vibrational modes are not occupied. This would lead to an experimentally lower reaction rate value when compared to the reaction rate value predicted by transition state theory.
Good. Just be aware that we only have 3 atoms in our system, so there is no Boltzmann distributionSw2711 (talk) 17:08, 16 May 2019 (BST)
Excercise 2: F-H-H System
F + H2 and H + HF Reactions
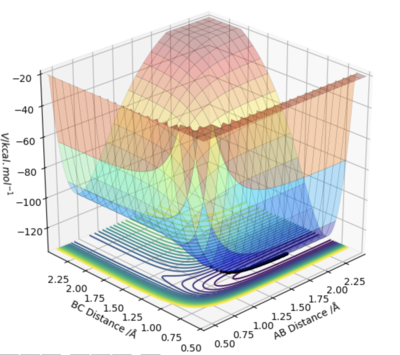
A surface plot for the reactions was plotted as can be seen in figures 6. The second reaction is just the reverse of the first reaction so the same surface plot can be used. By looking at the relative potential energy values for the start and end of each reaction, the change in energies associated with each reaction were determined. The reaction in which HF is formed was determined to be exothermic and the reverse reaction in which H2 is formed was deemed to be endothermic. In figure 6, HF is associated with the lower plateau and H2 with the higher one. The energy changes in the reaction occur since HF has a stronger bond than H2. This results in energy being released when HF is formed (net energy released by HF bond formation is greater than used to break the H2 bond) and energy being taken in when HF is being used (energy required to break HF bond is greater than the energy produced upon the formation the H2 bond).
Good.Sw2711 (talk) 17:09, 16 May 2019 (BST)
F-H-H Transition State
By invoking Hammond's postulate, and examining the surface plot, the transition state (which will be the same in both cases) will have a structure similar to that of H2+F. Through trial and error a good approximation of the transition state internuclear distances were found to be H-H = 0.745 and H-F = 1.808. A plot of internuclear distances is shown in figure 7.
Good. But it's better to demonstrate your strategy for your number of trials to get those values. And remember the unitsSw2711 (talk) 17:10, 16 May 2019 (BST)
Activation Energies Required for the Reactions
The activation energies were calculated by finding the difference between the energy of the transition state and the energy of the reactants. Using the transition state parameters calculated above the energy of the transition state was worked out to be -103.75. The energy of the energy of the reactants H2 + F was found to be -103.93, and the energy of the reactants HF + H was -133.94. As a result the activation energies are 30.2 and 0.2 for the formation of H2 + F and HF + H respectively.
Good. Again, I know you understand it, but it's better to explain how to get the energy for reactants/products. What kind of settings did you use? And the units.Sw2711 (talk) 17:12, 16 May 2019 (BST)
Reaction Dynamics

In the reaction between H2 + F, heat is generated by the reaction. Energy is conserved however. As can be seen by figure 8, translational energy is converted into vibrational energy. The changes in energy could be probed via bomb calorimetry (in which the temperature changes are measureed) or via emission IR spectroscopy.
Good. Translational energy is converted to vibrational energy, but does that mean they are conserved? What about the potential energy then? And you need to explain a bit more about the experimental techniques you suggested.Sw2711 (talk) 17:14, 16 May 2019 (BST)
The reaction in which HF + H was formed, was further investigated with the following parameters: rHH = 0.74, pFH = -0.5 and rFH = 2. The pHH was varied in the range -3 to 3. As can be seen from the table above, the reaction is only successful for some pHH values.
| pHH | Reactive? |
|---|---|
| -3 | No |
| -2.95 | No |
| -2.9 | No |
| -2.85 | No |
| -2.5 | No |
| -2 | Yes |
| -1.5 | Yes |
| -1 | Yes |
| -0.5 | Yes |
| 0 | Yes |
| 0.1 | No |
| 0.5 | No |
| 1.0 | No |
| 1.5 | No |
| 2.0 | No |
| 2.5 | No |
| 2.85 | Yes |
| 2.9 | Yes |
| 2.95 | Yes |
| 3.0 | No |

However, if the pFH is changed to -0.8 and the pHH = 0.1, the reaction is successful. This shows that by increasing the kinetic energy of the system the reaction outcome changes from being unsuccessful (see table above, pHH = 0.1) to being successful.
The endothermic reaction in which H2 + F is formed is possible despite H-F having such a strong bond strength. It is possible if the parameters are set to: rHH = 2, pHH = -0.01 and rHF = 0.913 and pHF = -10. The reaction pathway can be seen in figure 9. In order for the reaction to be successful a high vibrational energy is required.
The above are examples of Polanyi's rules. For exothermic reactions with an early transition state, the success of a reaction is more likely given a higher translational energy in the system. This can be seen in the above example where changing pFH from -0.5 to -0.8 (i.e. increasing the kinetic energy of the system) meant the reaction when pHH = 0.1 went from being unsuccessful to being successful. If the transition state is late however, the reaction efficiency is increased with increasing vibrational energies.
This part is good piece of work.Sw2711 (talk) 17:16, 16 May 2019 (BST)
References
(1) Atkins, P. and De Paula, J. (n.d.). Physical chemistry. 7th ed.